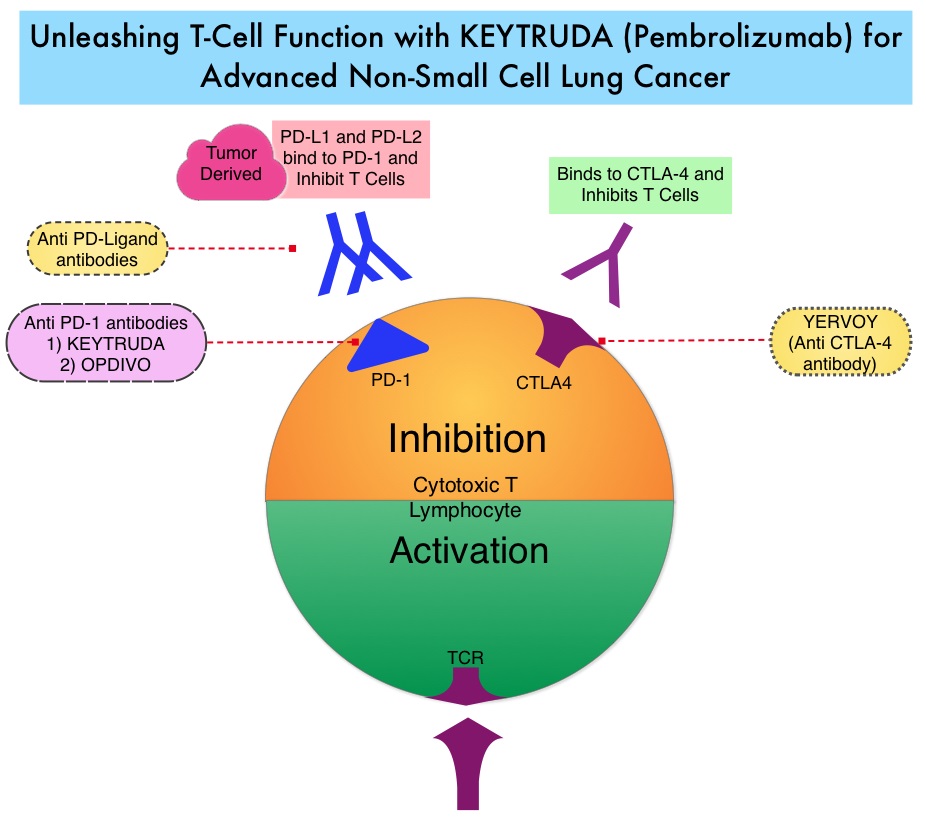
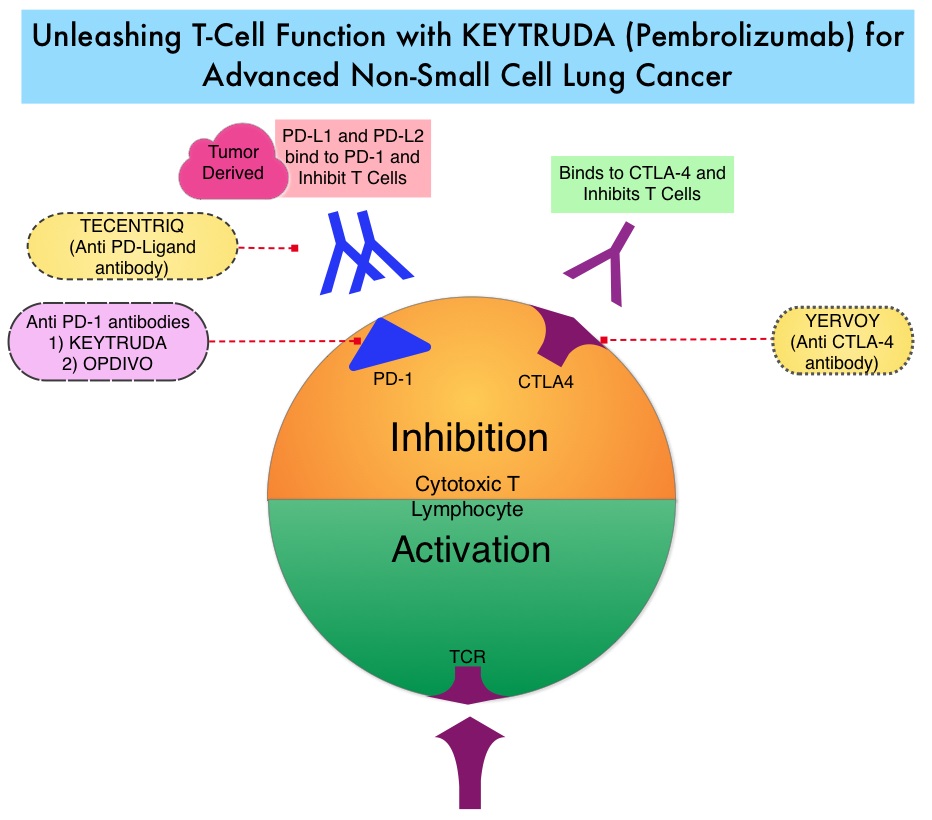
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Patients with advanced NSCLC (Non-Small Cell Lung Cancer) often receive either platinum-doublet chemotherapy combination as first line therapy or KEYTRUDA® (Pembrolizumab) if the tumor PD-L1 expression is 50% or more. About 20-25% of patients benefit from immunotherapy. Other biomarkers besides PD-L1 are needed, to select appropriate patients for immunotherapy.
Tumor Mutational Burden (TMB) has recently emerged as a potential biomarker for immunotherapy with anti PD-1 antibodies. TMB can be measured using Next-Generation Sequencing (NGS) and is defined as the number of somatic, coding base substitutions and short insertions and deletions (indels) per megabase of genome examined. In a previously published trial (CheckMate 568), patients most likely to have a response to a combination of OPDIVO® (Nivolumab) plus YERVOY® (Ipilimumab), irrespective of tumor PD-L1 expression level in NSCLC, had a TMB of at least 10 mutations per megabase. This was the basis for CheckMate 227, which evaluated the efficacy of OPDIVO® and OPDIVO®-based regimens, as first line treatment in biomarker-selected groups of patients with advanced NSCLC.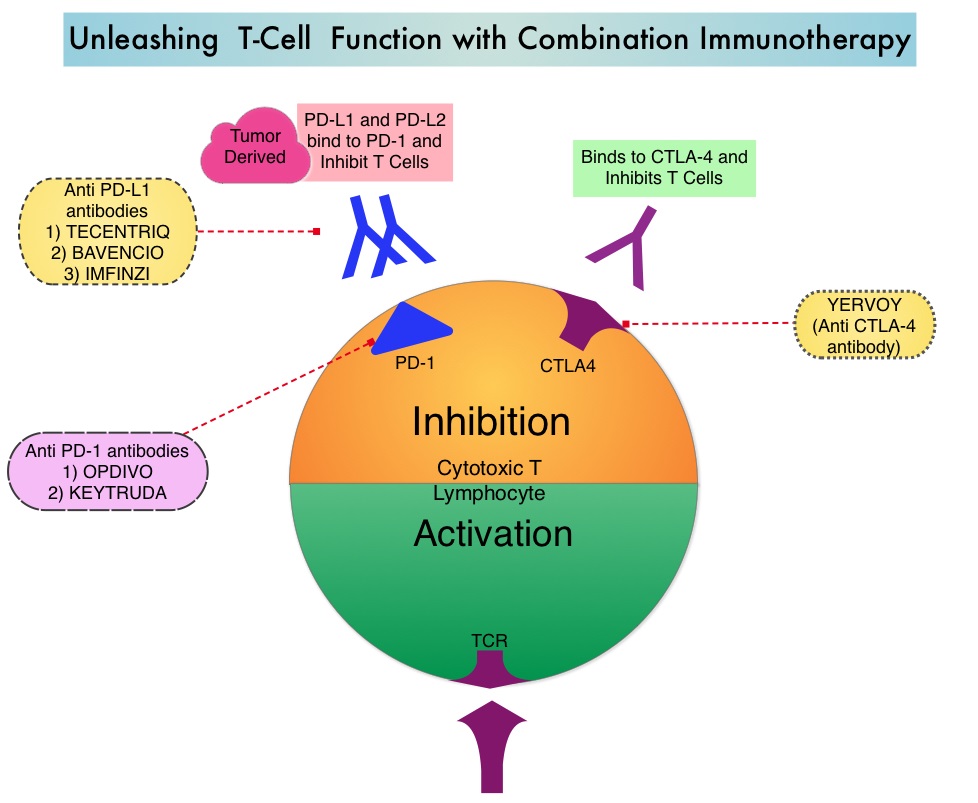
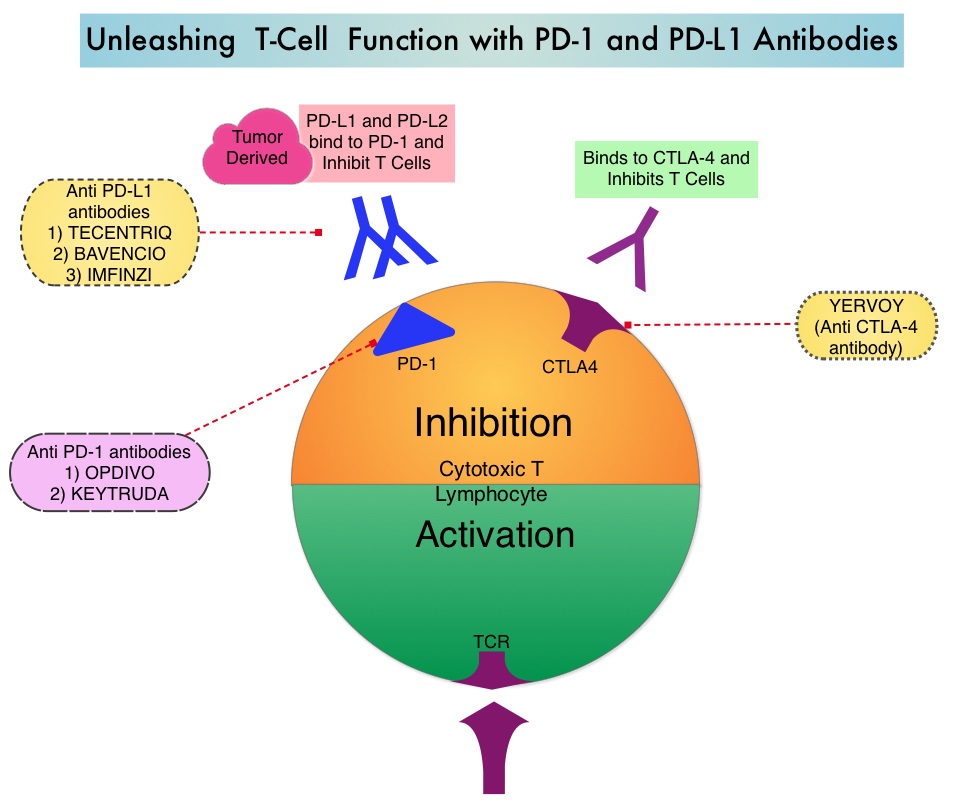
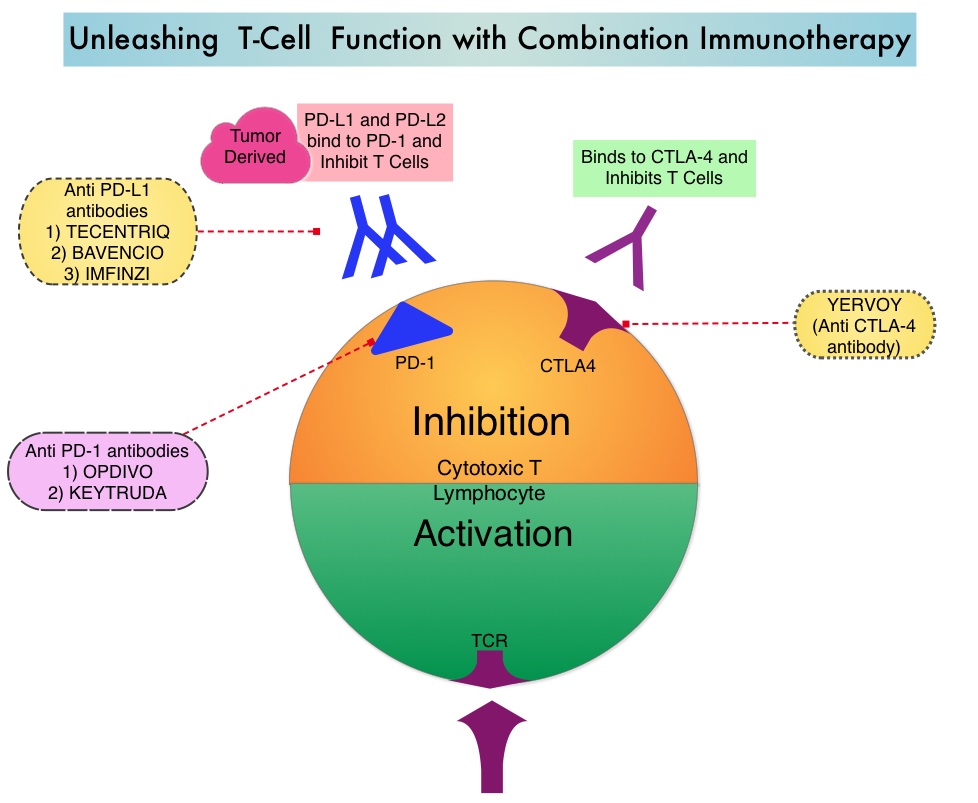
OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, whereas YERVOY® is a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4(Cytotoxic T-Lymphocyte Antigen 4, also known as CD152). Blocking the Immune checkpoint proteins unleashes the T cells, resulting in T cell proliferation, activation and a therapeutic response. The complementary mechanisms of action of OPDIVO® and YERVOY® combination resulted in greater efficacy in phase I trials, compared with OPDIVO® monotherapy.
CheckMate 227 is a three part, open-label, randomized, phase III trial, designed to compare different OPDIVO® -based regimens with chemotherapy in distinct patient populations. This study enrolled 1,739 patients with previously untreated Stage IV or recurrent NSCLC with no known sensitizing EGFR or ALK mutations and patients were randomized in a 1:1:1 ratio and the comparison was between either OPDIVO®, OPDIVO® plus YERVOY® or OPDIVO® plus platinum-doublet chemotherapy and platinum-doublet chemotherapy alone. Patients were stratified according to tumor histology and PD-L1 expression of 1% or more (positive) or less than 1% (negative). The study incorporated Tumor Mutational Burden (TMB) as a biomarker. This was determined by the FoundationOne CDx assay, an FDA approved test for molecular profiling, using the validated cutoff of TMB of 10 or more mutations/megabase as High, and less than 10 mutations/megabase as Low.
The authors in this publication reported data from part 1 of this study, which was a comparison between OPDIVO® plus YERVOY® versus chemotherapy, in patients with previously untreated Stage IV or recurrent NSCLC. In this comparison, 139 TMB-High patients were treated with OPDIVO® 3 mg/kg IV every 2 weeks plus YERVOY® 1 mg/kg IV every 6 weeks, whereas 160 TMB-High patients received chemotherapy, based on tumor histology. All treatments were continued until disease progression or unacceptable toxicity. Part 1 of the study had two Coprimary end points. One Coprimary end point was Progression Free Survival (PFS) with OPDIVO® plus YERVOY® versus chemotherapy in a patient population selected on the basis of TMB. The other Coprimary end point was Overall Survival (OS) with OPDIVO® plus YERVOY® versus chemotherapy in a patient population selected on the basis of the PD-L1 expression level.
It was noted that the PFS among patients with High TMB was significantly longer with OPDIVO® plus YERVOY®, compared with chemotherapy. The median PFS with the immunotherapy combination was 7.2 months compared to 5.5 months with chemotherapy (HR=0.58; P<0.001). This represented a 42% reduction in the risk of disease progression or death. The 1 year PFS more than tripled with combination immunotherapy at 42.6% versus 13.2% with chemotherapy. The Objective Response Rate (ORR) was 45.3% with immunotherapy combination and 26.9% with chemotherapy. The improved outcomes with OPDIVO® plus YERVOY® over chemotherapy was broadly consistent within all subgroups and was independent of tumor histology and PD-L1 expression. There was a clear trend toward improved survival with the immunotherapy combination although this data is immature. Grade 3 or 4 treatment related adverse events were 31.2% with immunotherapy combination and 36.1% with chemotherapy.
The authors concluded that this is the first phase III study to evaluate Tumor Mutational Burden as a predictive biomarker for immunotherapy as a coprimary endpoint. They added that these results highlight that Tumor Mutational Burden and PD-L1 are independent biomarkers and TMB is predictive of benefit with OPDIVO® plus YERVOY® irrespective of PD-L1 expression. TMB-High therefore is a new biomarker and represents a distinct subgroup of NSCLC. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. N Engl J Med 2018; 378:2093-2104

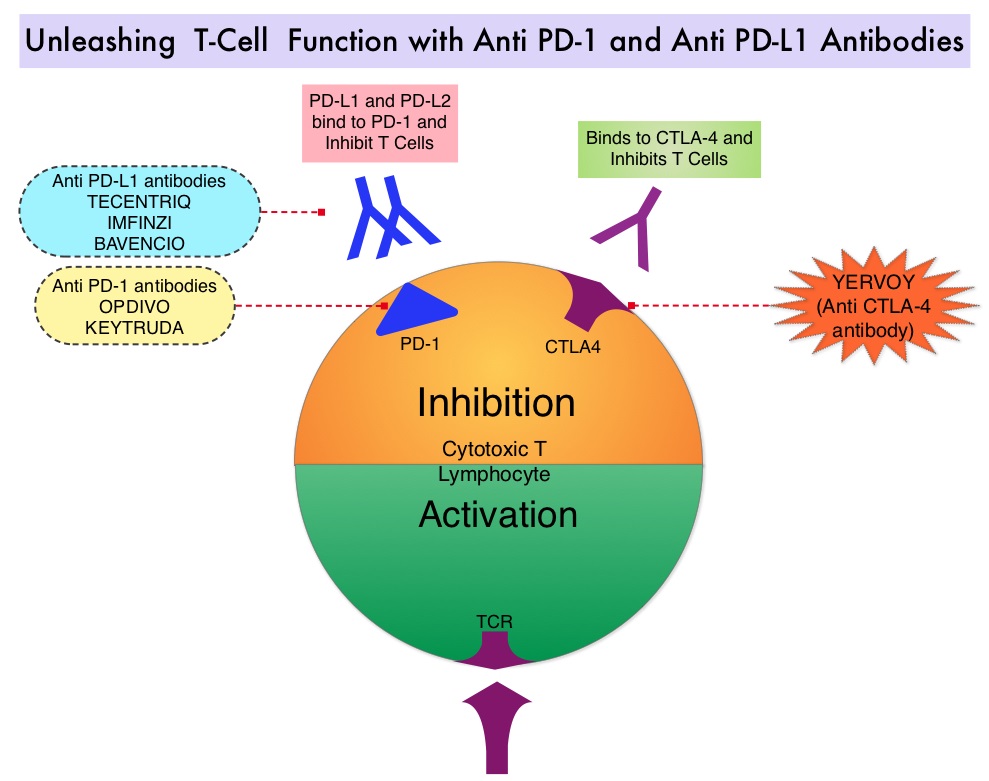
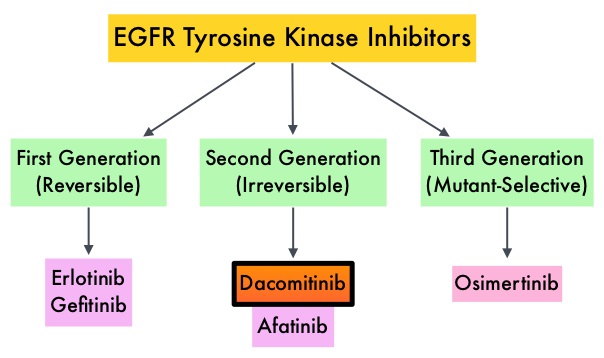 EGFR-Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60-70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9-14 months. T
EGFR-Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60-70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9-14 months. T