SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas, and 10% are Large cell carcinomas. Non Small Cell Lung Cancer patients with Squamous Cell histology have been a traditionally hard- to-treat, patient group, and less than 15% of patients with advanced Squamous NSCLC survive a year after diagnosis and less than 5% of patients survive for five years or longer. Immunotherapy is an accepted second line intervention after Platinum-based chemotherapy, in patients with advanced NSCLC, and is an approved first line therapy, for patients with high PD-L1 expressing tumors (50% or more).
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody, designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, thereby blocking its interactions with PD-1 and B7.1 receptors and thus enabling the activation of T cells. TECENTRIQ® was approved by the FDA in October 2016 for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC) whose disease progressed during or following Platinum-containing chemotherapy. In this present publication, the authors studied the efficacy of TECENTRIQ® given along with combination chemotherapy, in patients with advanced Squamous NSCLC.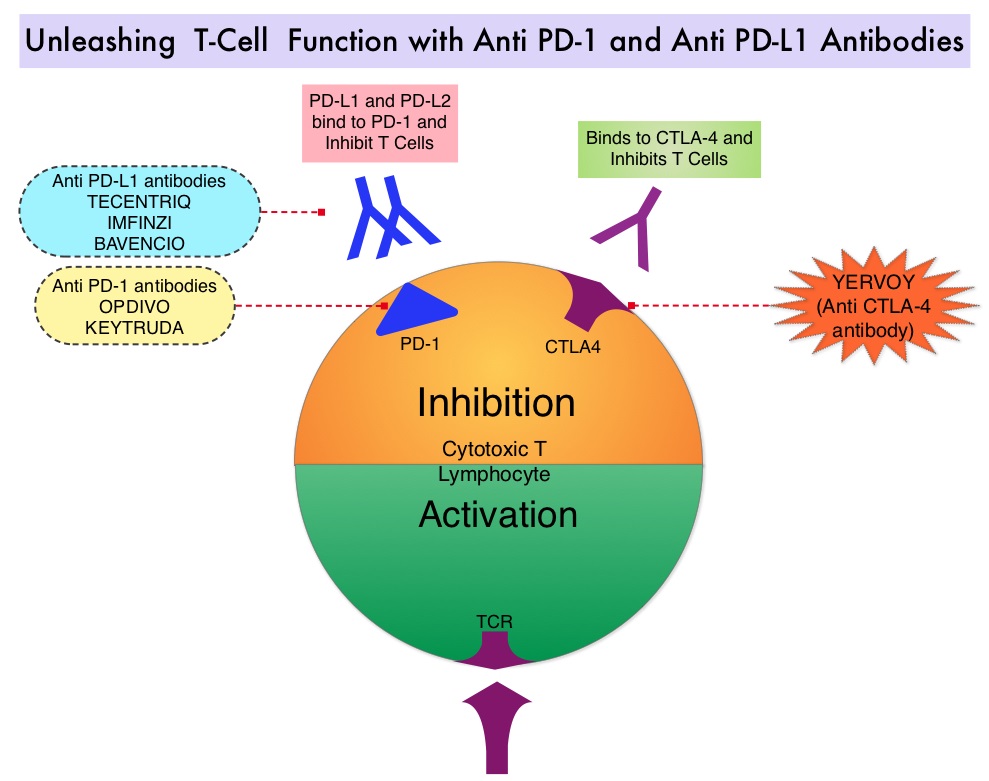
IMpower131 is a multicenter, open-label, phase III study, in which 1021 chemotherapy-naïve patients with stage IV Squamous NSCLC were randomly assigned in 1:1:1 ratio to receive TECENTRIQ® along with Carboplatin, and Paclitaxel (Group A, N=338), TECENTRIQ® along with Carboplatin, and ABRAXANE® (nab-paclitaxel) (Group B, N=343) and the control arm of Carboplatin and ABRAXANE® (Group C, N=340). Patients in Group A received TECENTRIQ® 1200 mg IV along with Carboplatin AUC 6 and TAXOL® (Paclitaxel) 200 mg/m2 IV, all drugs given on Day 1, every 21 days. Patients in Group B received TECENTRIQ® 1200 mg IV along with Carboplatin AUC 6 IV on Day 1 and ABRAXANE® 100mg/m2 IV on Days 1, 8, and 15 of each 21-day cycle. Patients in Group C (control group) received Carboplatin AUC 6 IV on Day 1 and ABRAXANE® 100mg/m2 IV on Days 1, 8, and 15 of each 21-day cycle. Patients received 4-6 cycles of this combination treatment and in Groups A and B, TECENTRIQ® alone was continued as long as there was a clinical benefit, without evidence of disease progression. Tumors were tested for PD-L1 expression, but patients were included in the study regardless of PD-L1 expression level. Patients with tumors demonstrating EGFR or ALK gene changes should have received molecularly targeted treatments before enrolling in this study. The co-Primary endpoints for this study were Progression Free Survival (PFS) and Overall Survival (OS). As per the study design, the current analysis compared the outcomes of patients in Group B with Group C. Outcomes data comparing Group A with Group C are not yet available.
At the time of primary analysis, with a median follow up of 17.1 months, the median PFS across all PD-L1 subgroups was 6.3 months with the addition of TECENTRIQ® to chemotherapy (Group B) versus 5.6 months in Group C, with chemotherapy alone (HR=0.71; P=0.0001). This represented a 29% reduction in the risk of disease progression or death, with the addition of TECENTRIQ® to chemotherapy. The 12-month PFS rates in Groups B and C were 24.7% versus 12.0%, respectively, suggesting a doubling of PFS benefit with the addition of TECENTRIQ® to chemotherapy. The PFS benefit was more pronounced in those with higher tumor PD-L1 expression. Overall Survival data are not yet mature. The most common side effects with the addition of TECENTRIQ® to chemotherapy included skin rash, colitis, and hypothyroidism.
The authors concluded that this is the first phase III trial of an immunotherapy-based treatment regimen, to demonstrate a significant improvement in Progression Free Survival, in advanced Squamous NSCLC. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. Jotte RM, Cappuzzo F, Vynnychenko I, et al. J Clin Oncol 36, 2018 (suppl; abstr LBA9000)

