The FDA on February 16, 2018 approved IMFINZI® for patients with unresectable stage III Non-Small Cell Lung Cancer (NSCLC,) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy. IMFINZI® is a product of AstraZeneca Inc.
Tag: Lung Cancer: Non-Small Cell
Long Term Survival Outcomes with OPDIVO® in Advanced NSCLC
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Under normal circumstances, Immune checkpoints or gate keepers inhibit intense immune responses by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.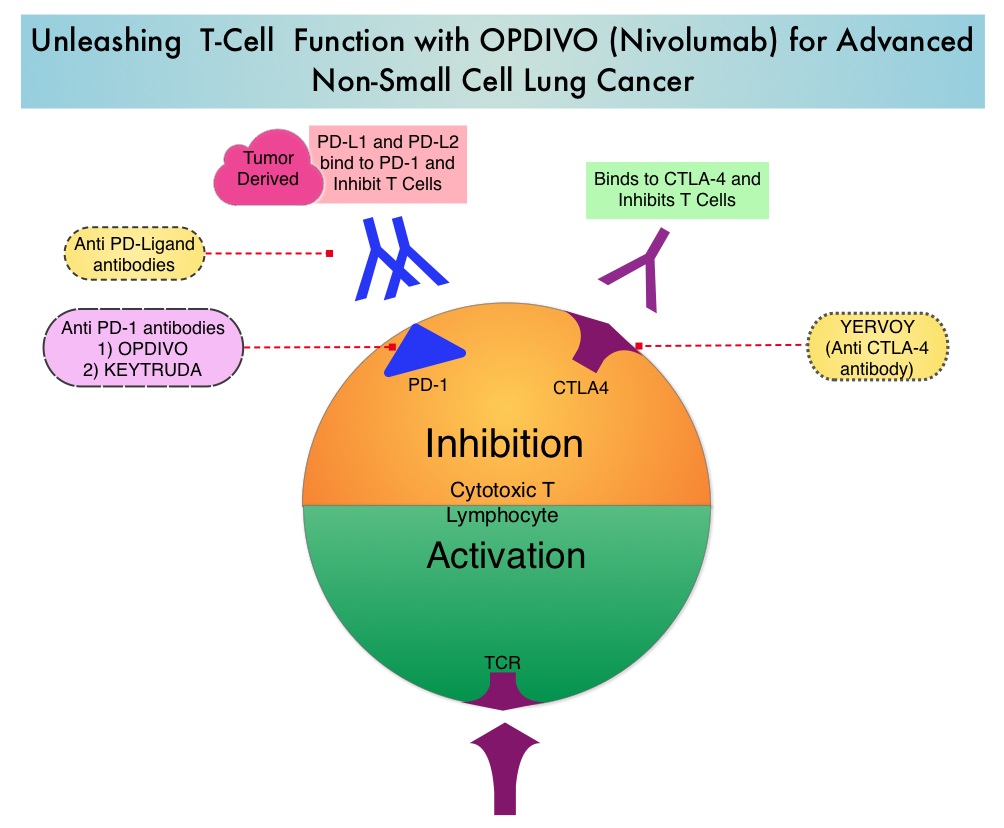
OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® significantly prolonged Overall Survival (OS) and had a favorable toxicity profile, when compared to TAXOTERE® (Docetaxel), in two open-label, randomized, phase III trials, among patients with advanced squamous (CheckMate 017) or non-squamous (CheckMate 057) NSCLC, who had disease progression during or after platinum-based chemotherapy. This data led to the approval of OPDIVO® in previously treated advanced NSCLC.
There is however limited data on long term efficacy and safety for immune checkpoint inhibitors in patients with NSCLC when compared with chemotherapy. The authors in this publication reported updated efficacy and safety data for OPDIVO® in patients with advanced NSCLC from the CheckMate 017 and CheckMate 057 trials, with a minimum follow up of 2 years in all patients. Patients in both trials had stage IIIB or IV disease and had disease progression during or after platinum-based chemotherapy. In CheckMate 017 study, 272 patients with metastatic squamous NSCLC were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). In CheckMate 057 trial, 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (N=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (N=290). Treatment was continued until disease progression or unacceptable toxicity. The minimum follow up for survival was 24.2 months.
The Overall Survival rate at 2 years with OPDIVO® versus TAXOTERE® were 23% vs 8% among squamous NSCLC patients and 29% vs 16% among non-squamous NSCLC patients. The relative reductions in the risk of death with OPDIVO® versus TAXOTERE® was similar to what was reported in the primary analyses. As was reported at the time of the primary analysis of these two trials, the superiority of OPDIVO® over TAXOTERE® was independent of Programmed cell Death Ligand 1 (PD-L1) expression in squamous NSCLC, whereas among patients with non-squamous histology, the benefit with OPDIVO® was greater in those with higher levels of PD-L1 expression, but patients with PD-L1 expression of less than 1% benefited as well.
In the pooled analysis of both trials, the median OS was 11.1 months with OPDIVO® versus 8.1 months with TAXOTERE® (HR=0.72). The relative reduction in the risk of death with OPDIVO® was 28%. Higher PD-L1 expression levels was associated with greater OS benefit with OPDIVO® (HR=0.42 in patients with 50% or more PD-L1 expression). Survival benefit however was still observed in patients with 1% or less PD-L1 expression (HR=0.78). Durable responses were noted with OPDIVO® among 37% of the confirmed responders with squamous NSCLC and 34% of confirmed responders with non-squamous NSCLC. These patients had ongoing responses after a minimum follow up of 2 years whereas no patient in the TAXOTERE® treated group had an ongoing response. The rates of treatment-related adverse events of any grade were lower with OPDIVO® compared to TAXOTERE® (any grade: 68% vs 88%; grade 3-4: 10% vs 55%, respectively).
The authors concluded that OPDIVO® provides long term clinical benefit and has a favorable tolerability profile compared with TAXOTERE®, in previously treated patients with advanced NSCLC. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non–Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). Horn L, Spigel DR, Vokes EE, et al. J Clin Oncol 2017;35:3924-3933
GILOTRIF® (Afatinib)
The FDA on January 12, 2018 granted approval to GILOTRIF® for a broadened indication in first-line treatment of patients with metastatic Non-Small Cell Lung Cancer (NSCLC) whose tumors have non-resistant Epidermal Growth Factor Receptor (EGFR) mutations, as detected by an FDA-approved test. GILOTRIF® is a product of Boehringer Ingelheim Pharmaceutical, Inc.
Molecular Testing in Lung Cancer – Guideline Update
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
The College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology convened an expert panel in 2013 and had published evidence-based guideline to set standards for the molecular analysis of lung cancers and to guide treatment decisions with targeted therapies. With the availability of new medical information and technological advances, this expert panel which comprised of pathologists, oncologists, pulmonologists, and laboratory scientists, issued an evidence based update which included 18 new recommendations, along with 3 updated recommendations from the 2013 guideline, asking 5 key questions.
Key Question 1: Which new genes should be tested for lung cancer patients?
a) ROS1 testing must be performed on all lung adenocarcinoma patients, irrespective of clinical characteristics.
b) ROS1 ImmunoHistoChemistry (IHC) may be used as a screening test in lung adenocarcinoma patients; however, positive ROS1 IHC results should be confirmed by a molecular or cytogenetic method.
c) BRAF, RET, ERBB2 (HER2), KRAS and MET molecular testing are currently not indicated as a routine stand-alone assay, outside the context of a clinical trial. It is appropriate to include molecular testing for these genes, as part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS1 testing are negative.
Key Question 2: What methods should be used to perform molecular testing?
a) ImmunoHistoChemistry (IHC) is an equivalent alternative to Fluorescence In Situ Hybridization (FISH) for ALK testing.
b) Multiplexed genetic sequencing panels are preferred over multiple single-gene tests, to identify other treatment options beyond EGFR, ALK, and ROS1.
c) Laboratories should ensure test results that are unexpected, discordant, equivocal or otherwise of low confidence, are confirmed or resolved, using an alternative method or sample.
Key Question 3: Is molecular testing appropriate for lung cancers that do not have an adenocarcinoma component?
a) Physicians may use molecular biomarker testing in tumors with histologies other than adenocarcinoma when clinical features indicate a higher probability of an oncogenic driver.
Key Question 4: What testing is indicated for patients with targetable mutations who have relapsed on targeted therapy?
a) In lung adenocarcinoma patients who harbor sensitizing EGFR mutations and have progressed after treatment with an EGFR-targeted TKI, physicians must use EGFR T790M mutational testing when selecting patients for third-generation EGFR-targeted therapy.
b) Laboratories testing for EGFR T790M mutation in patients with secondary clinical resistance to EGFR-targeted kinase inhibitors should deploy assays capable of detecting EGFR T790M mutations in as little as 5% of viable cells.
c) There is currently insufficient evidence to support a recommendation for or against routine testing for ALK mutational status for lung adenocarcinoma patients with sensitizing ALK mutations, who have progressed after treatment with an ALK-targeted Tyrosine Kinase Inhibitor (TKI).
Key Question 5: What is the role of testing for circulating cell-free DNA for lung cancer patients?
a) There is currently insufficient evidence to support the use of circulating cfDNA molecular methods for the diagnosis of primary lung adenocarcinoma.
b) In some clinical settings in which tissue is limited and/or insufficient for molecular testing, physicians may use a cfDNA assay to identify EGFR mutations.
c) Physicians may use cfDNA methods to identify EGFR T790M mutations in lung adenocarcinoma patients with progression or secondary clinical resistance to EGFR-targeted TKI; testing of the tumor sample is recommended if the plasma result is negative.
d) There is currently insufficient evidence to support the use of circulating tumor cell molecular analysis for the diagnosis of primary lung adenocarcinoma, the identification of EGFR or other mutations, or the identification of EGFR T790M mutations at the time of EGFR TKI resistance.
2013 Statements VERSUS 2017 Statements
a) 2013 – Cytologic samples are also suitable for EGFR and ALK testing, with cell blocks being preferred over smear preparations VERSUS 2017 – Pathologists may use either cell blocks or other cytologic preparations as suitable specimens for lung cancer biomarker molecular testing.
b) 2013 – Laboratories should use EGFR test methods that are able to detect mutations in specimens with at least 50% cancer cell content, although laboratories are strongly encouraged to use (or have available at an external reference laboratory) more sensitive tests that are able to detect mutations in specimens with as little as 10% cancer cells VERSUS 2017 – Laboratories should use, or have available at an external reference laboratory, clinical lung cancer biomarker molecular testing assays that are able to detect molecular alterations in specimens with as little as 20% cancer cells.
c) 2013 – IHC for total EGFR is not recommended for selection of EGFR TKI therapy VERSUS 2017 – It is strongly recommended that laboratories should not use total EGFR expression by IHC testing to select patients for EGFR-targeted TKI therapy.
Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Lindeman NI, Cagle PT, Aisner DL et al. [published online January 22,2018]. Arch Pathol Lab Med . doi: 10.5858/arpa.2017-0388-CP
KEYTRUDA® Doubles Overall Survival Compared with Chemotherapy in Advanced NSCLC
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers.
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor (immune checkpoint protein) and blocks its interaction with ligands PD-L1 and PD-L2. This leads to the undoing of the PD-1 pathway-mediated inhibition of the immune response and the tumor-specific effector T cells are unleashed. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced Non Small Cell Lung Cancer (NSCLC) have a high level of PD-L1 expression and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®.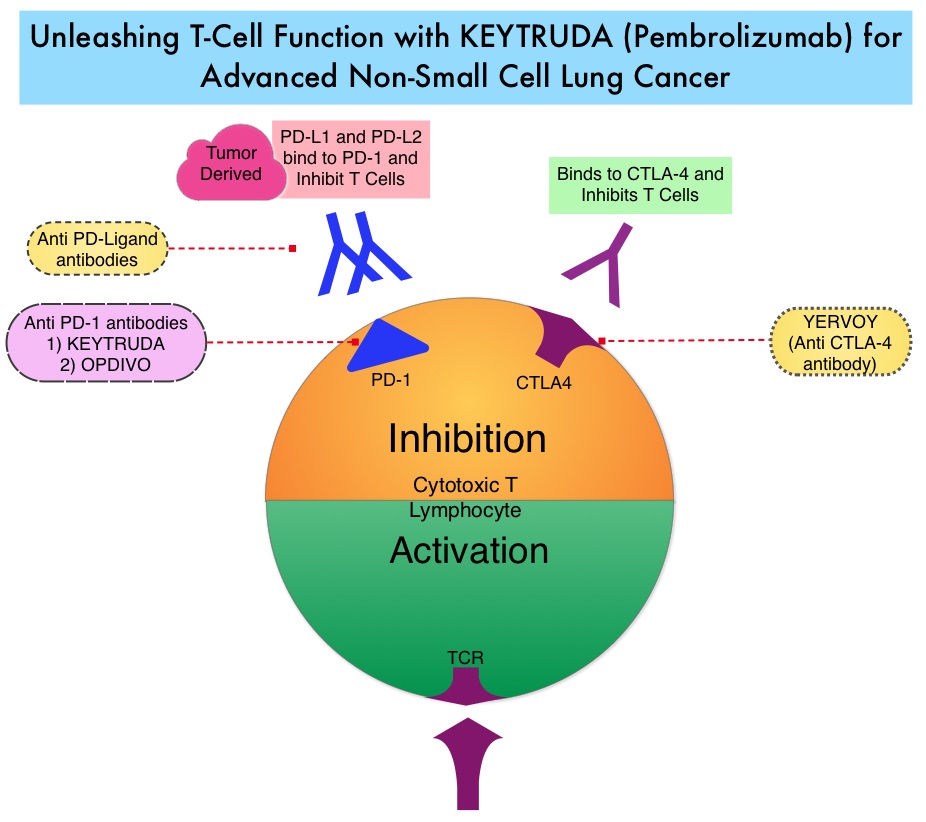
KEYNOTE-024 is an open-label, randomized phase III trial in which KEYTRUDA® administered at a fixed dose was compared with investigator’s choice of cytotoxic chemotherapy, as first line therapy, for patients with advanced NSCLC, with tumor PD-L1 expression of 50% or greater. Three hundred and five (N=305) treatment naïve patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, were randomly assigned in a 1:1 ratio to receive either KEYTRUDA® (N=154) or chemotherapy (N=151). Enrolled patients had no sensitizing EGFR mutations or ALK translocations. Treatment consisted of KEYTRUDA® administered at a fixed dose of 200 mg IV every 3 weeks for 35 cycles or the investigator’s choice of platinum-based chemotherapy for 4-6 cycles. Pemetrexed (ALIMTA®) based therapy was permitted only for patients who had non-squamous tumors and these patients could receive ALIMTA® maintenance therapy after the completion of combination chemotherapy. Patients in the chemotherapy group who experienced disease progression were allowed to cross over to the KEYTRUDA® group. The primary end point was Progression Free Survival (PFS) and secondary end points included Overall Survival (OS), Objective Response Rate (ORR) and safety.
It was previously reported that at a median follow up of 11.2 months, the median PFS was 10.3 months in the KEYTRUDA® group versus 6 months in the chemotherapy group (HR=0.50; P<0.001). However, median OS had not been reached in the KEYTRUDA® group at the time of that analysis. This publication is an updated analysis of the KEYNOTE-024 study, after a median follow-up of 25.2 months. Eighty two patients (N=82) assigned to chemotherapy, met criteria to cross over to the KEYTRUDA® group, upon progression. The median OS was 30 months in the KEYTRUDA® group and 14.2 months in the chemotherapy group (HR=0.63). Further, more patients in the KEYTRUDA® group achieved 12-month OS (70.3% vs. 54.8%) and an ORR response (45.5% vs. 29.8%), compared to the chemotherapy group. The ORR among patients who crossed over to KEYTRUDA®, was 20.7%. The median Duration of Response has not yet been reached for patients assigned to KEYTRUDA® and also for those who crossed over to KEYTRUDA®. For those assigned chemotherapy, the median Duration of Response was 7.1 months. Patients in the KEYTRUDA® group had lower rates of grade 3 to 5 adverse events compared to those in the chemotherapy group (31.2% vs 53.3%), as well as a lower rate of any-grade adverse events (76.6% vs 90%).
It was concluded that first-line treatment with KEYTRUDA® resulted in a significantly longer median OS with lower rates of Adverse Events, when compared to chemotherapy, among patients with metastatic NSCLC and high PD-L1 expression. Brahmer JR, Rodriguez-Abreu D, Robinson A, et al. Updated analysis of KEYNOTE-024: pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS>50%. Presented at: International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer; Yokohama, Japan: October 15-18, 2017. Abstract OA 17.06.
Consolidation with IMFINZI® after Chemoradiotherapy Improves Outcomes in Patients with Unresectable Stage III Non-Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Approximately one third of all patients with NSCLC have stage III, locally advanced disease at the time of initial presentation. Worldwide, about 500,000 patients are diagnosed with unresectable, stage III NSCLC, each year. These patients include those with locally advanced primary tumors with tumor invading the vital mediastinal organs, as well as those with involvement of locoregional mediastinal lymph nodes. These patients are often treated with platinum-based doublet chemotherapy with concurrent radiation and have a median Progression Free Survival (PFS) of approximately 8 months and 5 year survival of only 15%. There is hence a significant unmet need for this patient group, with no major treatment advances thus far.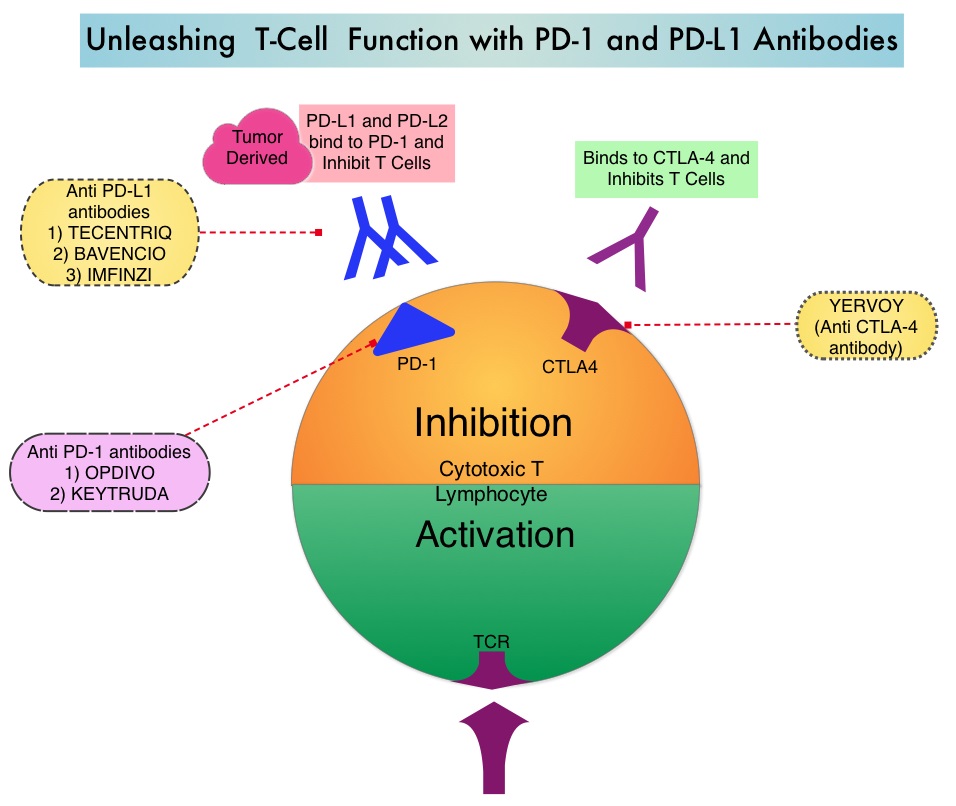
Preclinical evidence had suggested that chemotherapy and radiotherapy may upregulate PD-L1 expression in tumor cells. IMFINZI® (Durvalumab) is a selective, high-affinity, human IgG1 monoclonal antibody, that blocks the binding of Programmed Death Ligand 1 (PD-L1) to Programmed Death 1 (PD-1) and CD80, thereby unleashing the T cells to recognize and kill tumor cells. IMFINZI® showed encouraging antitumor activity in an early phase clinical study involving multiple advanced solid tumors, including stage IIIB or IV NSCLC. IMFINZI® was recently approved by the FDA for the treatment of patients with locally advanced or metastatic urothelial carcinoma, who had received prior platinum-based chemotherapy.
The authors in this publication evaluated the role of immune checkpoint blockade in locally advanced, unresectable, stage III NSCLC. PACIFIC trial is a randomized, double-blind, international, phase III study in which IMFINZI® as consolidation therapy was compared with placebo, in patients with stage III, locally advanced, unresectable NSCLC, that had not progressed following platinum-based chemoradiotherapy. Eligible patients received two or more cycles of platinum-based doublet chemotherapy concurrently with definitive radiation therapy (54-66 Gy). Following completion of concurrent chemoradiation treatment, 713 patients were randomized, of whom 709 patients in a 2:1 ratio received consolidation treatment, within 6 weeks after completion of chemoradiation with IMFINZI® 10 mg/kg every 2 weeks (N=473) or placebo (N=236), for up to 12 months. The median age was 64 years, and the majority of patients were men (70%) and 46% had a squamous histology. The coprimary end points were Progression Free Survival (PFS) and Overall Survival (OS). Secondary end points included 12-month and 18-month PFS rates, Objective Response Rate (ORR), Duration of Response, time to death or distant metastasis, and safety. The authors reported the results of a preplanned interim analysis after a median follow up of 14.5 months.
The median PFS from randomization to consolidation treatment was 16.8 months with IMFINZI® versus 5.6 months with placebo (HR=0.52; P<0.001). This meant a 48% decrease in the probability of disease progression with IMFINZI® and this improvement was consistent across all patient subgroups that were analyzed. The 12-month PFS was 55.9% vs 35.3%, and the 18-month PFS rate was 44.2% vs 27.0%, in favor of IMFINZI®. The ORR was higher with IMFINZI® compared to placebo (28.4% vs 16.0%; P<0.001), and the median Duration of Response was longer as well, with 73% of the patients in the IMFINZI® group having an ongoing response at 18 months versus 47% of the patients in the placebo group. Patients in the IMFINZI® group also had a lower incidence of new brain metastases. The median time to death or distant metastasis was longer with IMFINZI® compared with placebo (23.2 months vs 14.6 months; P<0.001). Adverse events of any grade occurred in 68% of patients in the IMFINZI® group compared to 53% in the placebo group and majority of the toxicities were grade 1 or 2, and grade 3 or higher toxicities were infrequent (less than10%), in both treatment groups. Treatment had to be discontinued due to pneumonitis in 6.3% of patients on IMFINZI® and 4.3% on placebo.
It was concluded that IMFINZI® significantly prolonged PFS in all prespecified groups of patients with locally advanced stage III NSCLC, and toxicity profile was acceptable. Biomarkers, such as mutational load or immunosignature, may be of value, as PD-L1 expression had little or no impact on outcomes. The National Comprehensive Cancer Network (NCCN) Guidelines have been updated to include one year of consolidation therapy with IMFINZI®, after curative-intent chemoradiation, for inoperable stage III lung cancer. Durvalumab after Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer. Antonia SJ, Villegas A, Daniel D, et al. for the PACIFIC Investigators. N Engl J Med 2017; 377:1919-1929
TAGRISSO® Superior to First Generation EGFR TKIs in Advanced Non-Small Cell Lung Cancer
Approximately 10% to 15% of Caucasian patients and 35-50% of Asian patients with Adenocarcinomas, harbor activating EGFR (Epidermal Growth Factor Receptor) mutations and 90% of these mutations are either Exon 19 deletions or L858R point mutations in Exon 21. EGFR-Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60% to 70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9 to 14 months. This resistance to frontline EGFR TKI therapy has been attributed to acquired T790M “gatekeeper” point mutation in EGFR, identified in 50% – 60% of patients.
TAGRISSO® (Osimertinib), is a third-generation Epidermal Growth Factor Receptor (EGFR) TKI and in a randomized, double blind, phase III clinical trial, demonstrated superior efficacy and tolerability compared to the Standard of Care, as first-line therapy in patients with advanced EGFR mutation positive NSCLC. This benefit was seen even in those with CNS metastases at study entry. These new finding are very likely to change the treatment paradigm for NSCLC patients whose tumors harbor EGFR mutations.
TAGRISSO® Superior to First Generation EGFR TKIs in Advanced Non-Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer. Approximately 10% to 15% of Caucasian patients and 35-50% of Asian patients with Adenocarcinomas, harbor activating EGFR (Epidermal Growth Factor Receptor) mutations and 90% of these mutations are either Exon 19 deletions or L858R point mutations in Exon 21. EGFR-Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60% to 70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9 to 14 months. This resistance to frontline EGFR TKI therapy has been attributed to acquired T790M “gatekeeper” point mutation in EGFR, identified in 50% – 60% of patients.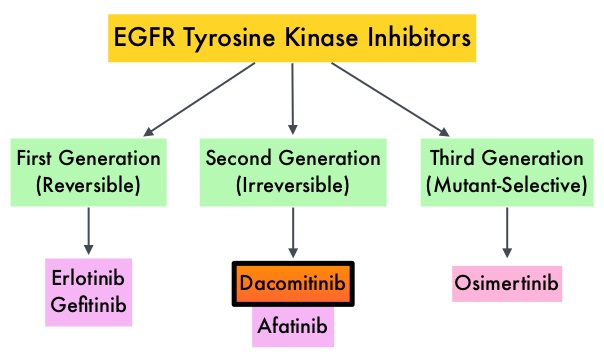
TAGRISSO® (Osimertinib), is a third-generation Epidermal Growth Factor Receptor (EGFR) TKI presently approved by the FDA, for the treatment of patients with metastatic EGFR T790M mutation-positive NSCLC, who had progressed on prior systemic therapy, including an EGFR-TKI. Previously published studies suggested that TAGRISSO® may also be effective as initial therapy for EGFR mutation-positive advanced NSCLC.
FLAURA is a randomized, double blind, phase III clinical trial, conducted to compare the efficacy and safety of first line TAGRISSO® to TARCEVA® or IRESSA® (which are considered Standard of Care as first line therapy), in NSCLC patients with activating mutations EGFR Exon 19 deletions or L858R substitution mutation on Exon 21. This study randomized 556 advanced NSCLC treatment naïve patients, with EGFR Exon 19 or 21 mutations in a 1:1 ratio, to TAGRISSO® 80 mg orally once daily (N=279) or Standard of Care EGFR-TKI, IRESSA® 250 mg or TARCEVA® 150 mg, orally once daily (N=277). Patients were stratified by mutation status (Exon 19 vs 21 mutations) and race (Asian vs non-Asian). Patients with CNS metastases who were neurologically stable, were allowed in this study. The Primary endpoint was Progression Free Survival (PFS).
The median PFS was 18.9 months with TAGRISSO® compared to 10.2 months for the standard therapy (HR=0.46; P<0.0001), suggesting a 54% reduction in the risk of disease progression, compared with Standard of Care. TAGRISSO® extended the median Time To Progression by about 9 months. This PFS benefit was consistent across all subgroups of patients, including those with and without CNS metastases at study entry. The Objective Response Rate (ORR) with TAGRISSO® was 80% compared with 76% for TARCEVA® and IRESSA®. The median Duration of Response with TAGRISSO® was 17.2 versus 8.5 months in the comparator arm. The median Overall Survival was not reached. Grade 3 and 4 toxicities were lower for TAGRISSO® (34%) compared with 45% for TARCEVA® and IRESSA®. Toxicities led to treatment discontinuation for 13% and 18% of patients in the TAGRISSO® and comparator groups, respectively.
It was concluded that TAGRISSO® demonstrated superior efficacy and tolerability compared to the Standard of Care, as first-line therapy in patients with advanced EGFR mutation positive NSCLC. Studies are underway, assessing treatments, following resistance to TAGRISSO®. Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFRm advanced NSCLC: FLAURA. Ramalingam S, Reungwetwattana T, Chewaskulyong B, et al. Presented at: 2017 ESMO Congress; Madrid, Spain; September 9-12, 2017. Abstract LBA2_PR.
ALECENSA® (Alectinib)
The FDA on November 6, 2017, granted regular approval to ALECENSA®, for treatment of patients with Anaplastic Lymphoma Kinase (ALK)-positive metastatic Non-Small Cell Lung Cancer (NSCLC), as detected by an FDA-approved test. ALECENSA® is a product of Hoffmann-La Roche, Inc./Genentech, Inc.
ASCO Clinical Practice Guideline Update for Stage IV Non Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. ASCO published the last clinical practice guideline update on systemic therapy for patients with Stage IV Non Small Cell Lung Cancer (NSCLC), in 2015. With the many advances in the management of these patients and availability of new practice changing evidence since the last publication, the latest ASCO guideline has been revised. The ASCO NSCLC Expert Panel updated their recommendations based on a systematic review of 14 randomized controlled trials from February 2014 to December 2016 and six nonrandomized studies on systemic therapy. This guideline is applicable to patients who had received molecular testing for EGFR/ALK/ROS1, as well as Programmed Death Ligand 1 (PD-L1), and clinicians know the test results.
Guideline Question: What systemic therapy treatment options should be offered to patients with Stage IV NSCLC, depending on the subtype of the patient’s cancer?
Target Population: Patients with Stage IV NSCLC.
Target Audience: Oncology care providers (including primary care physicians, specialists, nurses, social workers, and any other relevant member of a comprehensive multidisciplinary cancer care team), patients, and their caregivers.
Key Points:
1) There is no cure for patients with Stage IV NSCLC.
2) Decisions regarding chemotherapy should not be made based on age alone.
Recommendations: First Line Treatment for Patients
Patients with Non-Squamous Cell Carcinoma without a tumor EGFR-sensitizing mutation or ALK or ROS1 gene rearrangement and with a Performance Status (PS) of 0 or 1 (and appropriate PS of 2):
1) With high PD-L1 expression (Tumor Proportion Score [TPS] 50% or more) and no contraindications, single-agent Pembrolizumab is recommended.
2) With low PD-L1 expression (TPS less than 50%), a variety of combination cytotoxic chemotherapies (with or without Bevacizumab, if patients are receiving Carboplatin and Paclitaxel) are recommended.
3) There is insufficient evidence to recommend Bevacizumab in combination with Pemetrexed plus Carboplatin.
4) Other checkpoint inhibitors, combination checkpoint inhibitors, or immune checkpoint therapy with chemotherapy are not recommended.
5) With PS of 2, combination or single agent therapy or palliative care alone may be used.
Patients with Squamous Cell Carcinoma without a tumor EGFR-sensitizing mutation or ALK or ROS1 gene rearrangement and with a PS of 0 or 1 (and appropriate PS of 2):
1) With high PD-L1 expression (TPS 50% or more) and no contraindications, single agent Pembrolizumab is recommended.
2) With low PD-L1 expression (TPS less than 50%), a variety of combination cytotoxic chemotherapies are recommended.
3) Other checkpoint inhibitors, combination checkpoint inhibitors, or immune checkpoint therapy with chemotherapy are not recommended.
4) With PS of 2, combination or single agent therapy or palliative care alone may be used.
5) With Squamous NSCLC treated with Cisplatin and Gemcitabine, the Panel neither recommends for nor recommends against the addition of Necitumumab to chemotherapy.
With sensitizing EGFR mutations, Afatinib, Erlotinib, or Gefitinib is recommended.
With ALK gene rearrangements, Crizotinib is recommended.
With ROS1 rearrangement, Crizotinib is recommended.
Recommendations: Second Line Treatment for Patients
Without a tumor EGFR-sensitizing mutation or ALK or ROS1 gene rearrangement and with PS of 0 or 1 (and appropriate PS of 2):
1) In patients with high PD-L1 expression (TPS 1% or more), no contraindications, who received first line chemotherapy and have not received prior immune therapy, single agent Nivolumab, Pembrolizumab, or Atezolizumab is recommended.
2) In patients with negative or unknown tumor PD-L1 expression (TPS less than 1%), no contraindications and who received first line chemotherapy, single agent Nivolumab, or Atezolizumab, or a variety of combination cytotoxic chemotherapies are recommended.
3) Other checkpoint inhibitors, combination checkpoint inhibitors, and immune checkpoint therapy with chemotherapy are not recommended.
4) In patients who received an immune checkpoint inhibitor as first line therapy, a variety of combination cytotoxic chemotherapies are recommended.
5) In patients with contraindications to immune checkpoint inhibitor therapy after first line chemotherapy, Docetaxel is recommended.
6) In patients with non-Squamous Cell Carcinoma who have not previously received Pemetrexed, Pemetrexed is recommended.
With sensitizing EGFR mutations:
1) In patients with disease progression after first line therapy with an EGFR Tyrosine Kinase Inhibitor (TKI) and the presence of the T790M resistance mutation, Osimertinib is recommended. If T790M mutation is not present, a platinum doublet is recommended.
2) In patients who received an EGFR-TKI in the first-line setting, had an initial response, and subsequently experienced slow or minimal disease progression at isolated sites, EGFR-TKI with local therapy to the isolated sites is an option.
With ROS1 rearrangement:
1) In patients who have not received prior Crizotinib, Crizotinib is recommended.
2) In patients who have received prior Crizotinib, platinum-based therapy in the second line with or without Bevacizumab is recommended.
With BRAF mutations:
1) In patients without prior immune checkpoint therapy and high PD-L1 expression (TPS more than 1%), single agent Atezolizumab, Nivolumab, or Pembrolizumab is recommended.
2) In patients who have received prior immune checkpoint therapy, Dabrafenib alone or in combination with Trametinib in third line, is an option.
Recommendations: Third Line Treatment for Patients
1) In patients without a tumor EGFR-sensitizing mutation or ALK or ROS1 gene rearrangement and with non-Squamous Cell Carcinoma and PS of 0 or 1 (and appropriate PS of 2), who received chemotherapy with or without Bevacizumab and immune checkpoint therapy, single agent Pemetrexed or Docetaxel are options.
2) In patients with tumor EGFR-sensitizing mutation(s) who have received at least one first-line EGFR-TKI and prior platinum-based chemotherapy, there are insufficient data to recommend immunotherapy in preference to chemotherapy.
Recommendations: Fourth Line Treatment for Patients
Patients and clinicians should consider and discuss experimental treatment, clinical trials, and continued best supportive (palliative) care.
Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Hanna N, Johnson D, Temin S, et al. J Clin Oncol 2017;35:3484-3515
