SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas.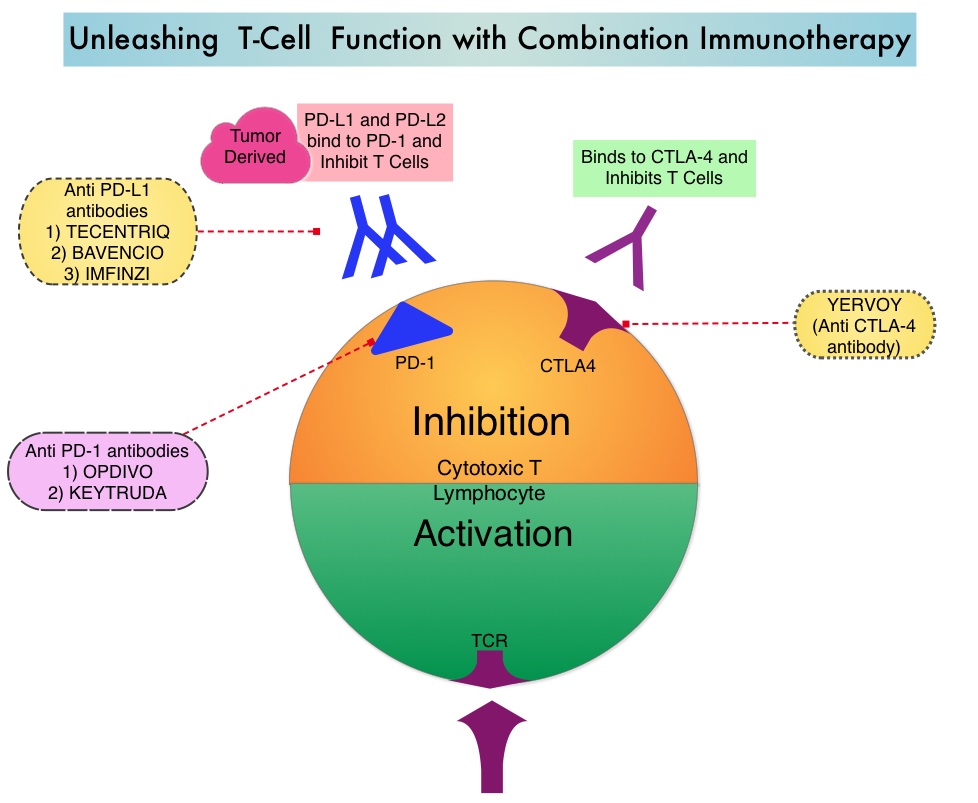
Immunotherapy with PD-1/PD-L1 (Programmed Death-1/Programmed Death-Ligand 1) inhibitors, also called Immune Checkpoint Inhibitors (ICIs), has changed the treatment paradigm for patients with advanced NSCLC. In previously treated patients with NSCLC, the Overall Response Rates (ORR) with single agent Immune Checkpoint Inhibitors (ICIs) range from 14-20%, with median Overall Survival (OS) of 10 to 12 months. In those with PD-L1 expression of 50% or more by ImmunoHistoChemical (IHC) analysis, the ORR can reach up to 30% with a median OS of 20 months. However, in patients with negative or weak PD-L1 expression (1%-49% positive tumor cells), who account for approximately two thirds of the NSCLC population, the response rates range from 8-19% with a median OS slightly below 10 months. Even among those with tumors expressing PD-L1 expression of 50% or more, not all patients benefit from Immunotherapy with ICIs. Therefore identifying biomarkers for patients likely to respond to ICI therapy, and predicting resistance is important and relevant in selecting the appropriate patients for treatment with ICIs.
There is growing evidence on the role of inflammation in cancer biology and systemic inflammatory response may have prognostic significance in different cancer types. Inflammatory process in various cancers imparts immunoresistance to ICIs, by activating oncogenic signaling pathways, there by promoting cancer growth and dissemination, with resulting poor outcomes. Derived Neutrophil-to-Lymphocyte ratio (dNLR) and serum Lactate DeHydrogenase (LDH) level have been investigated as potential inflammatory biomarkers in patients with cancer. The dNLR is calculated using a formula dNLR= Absolute Neutrophil Count/(White Blood Count – Absolute Neutrophil Count). These ratios are simple and easy to calculate from routine blood tests. Both these biomarkers have been correlated with Immune Checkpoint Inhibitor outcomes, in patients with melanoma. In two large studies involving patients with advanced melanoma treated with Ipilimumab and Pembrolizumab, dNLR of 3 or more and LDH of at least 2.5 times Upper Limit of Normal (ULN), reflected a pro-inflammatory status and resulted in poor outcomes.
Based on this important finding in malignant melanoma, the authors conducted a multicenter, retrospective study to determine whether combining the two factors – pretreatment dNLR and LDH (Lung Immune Prognostic Index-LIPI), was associated with resistance to ICIs in patients with advanced NSCLC. In this study, LIPI was developed on the basis of dNLR (derived Neutrophil-to-Lymphocyte Ratio) of greater than 3 and LDH greater than Upper Limit of Normal (ULN). LIPI was used to stratify patients with NSCLC into 3 groups (Good= 0 factors; Intermediate= 1 of 2 factors, Poor= 2 factors). The pooled cohort treated with ICIs included 466 patients with advanced NSCLC of whom 161 patients were treated at a single institution, to test the potential of the biomarkers score (test cohort) and the hypothesis was then validated in a larger multicentric validation cohort of 305 patients treated at 8 European academic centers. To determine whether the LIPI is specific to Immune Checkpoint Inhibitors (ICIs), a control cohort of 162 patients with advanced NSCLC, treated exclusively with chemotherapy, were also evaluated for LIPI. The median patient age was 62 years, 58% had Adenocarcinoma and 34% had Squamous histology, 74% had PD-L1 expression of at least 1% by IHC analysis and 26% had negative results. Median follow up was 12 months. The Primary end point was Overall Survival (OS) and Secondary end points included Progression Free Survival (PFS) and Disease Control Rate (DCR).
It was noted that in the test cohort, median PFS and OS were 3 and 10 months, respectively which is consistent with prior reports in patients with NSCLC, treated with PD-1 inhibitors in second or later lines. A dNLR greater than 3 and LDH greater than ULN were independently associated with OS. The median OS for Poor, Intermediate, and Good LIPI was 3 months, 10 months and 34 months respectively, and median PFS was 2 months, 3.7 months and 6.3 months respectively (P<0.001). This suggested that the population with a Poor (high) LIPI were more likely to have progressive disease as their best response to immunotherapy and had both shorter PFS and OS, compared to those with an Intermediate or Good (low) LIPI. Disease Control Rate (Complete plus Partial Response plus Stable disease) was also correlated with dNLR greater than 3 and LDH greater than ULN. These results were reproducible in the ICI validation cohort for OS, PFS, and DCR. LIPI however was not associated with outcome in patients treated with chemotherapy only, providing support that it might be a predictor of benefit from Immune Checkpoint Inhibitors (ICIs).
It was concluded that pretreatment LIPI, combining derived Neutrophil-to-Lymphocyte ratio (dNLR) greater than 3 and serum LDH level greater than Upper Limit of Normal, correlated with worse outcomes for Immune Checkpoint Inhibitors (ICIs). The authors suggested that this is the first study to explore LIPI in NSCLC and can serve as a potentially useful tool when selecting patients for treatment with Immune Checkpoint Inhibitors, and LIPI might be useful for identifying patients unlikely to benefit from treatment with an ICI. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. Mezquita L, Auclin E, Ferrara R, et al. JAMA Oncol. 2018;4:351-357

