SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers.
Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options, by improving Overall Response Rate and prolongation of survival, across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. Checkpoint inhibitors unleash the T cells resulting in T cell proliferation, activation, and a therapeutic response. Biomarkers predicting responses to ICI’s include Tumor Mutational Burden (TMB), Mismatch Repair (MMR) status, and Programmed cell Death Ligand 1 (PD‐L1) expression. Other biomarkers such as Tumor Infiltrating Lymphocytes (TILs), TIL‐derived Interferon‐γ, Neutrophil‐to‐Lymphocyte ratio, and peripheral cytokines, have also been proposed as predictors of response. The optimal duration of treatment with checkpoint inhibitors across tumor types is currently unknown and finding the balance between efficacy, toxicity and cost of therapy remains an ongoing challenge. There are presently no adequately powered, prospective, checkpoint inhibitor trials, comparing different treatment durations.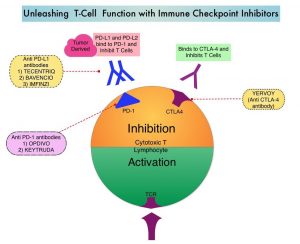
OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response, and unleashing the T cells. The authors in this study explored the impact of duration of treatment with OPDIVO®, on outcomes, in patients with previously treated advanced NSCLC, in a randomized study.
CheckMate 153 is a largely community based, ongoing, Phase IIIb/IV study, reflecting a real-world population, designed to evaluate the efficacy and safety of OPDIVO® monotherapy treatment duration, in previously treated advanced NSCLC. In this study, patients with previously treated advanced or metastatic NSCLC received OPDIVO® 3 mg/kg IV every 2 weeks until disease progression, unacceptable toxicity, or for 1 year. Treatment beyond initial progressive disease was permitted for patients with investigator-assessed clinical benefit, no rapidly progressive disease, and stable ECOG performance status, who were tolerating treatment. Patients who continued to receive treatment at 1 year were randomly assigned, regardless of response status to continue OPDIVO®, or to stop treatment (1-year fixed duration group), with the option of receiving OPDIVO® retreatment on study after disease progression. The Primary end point of safety was previously reported. Exploratory post-random assignment end points were added. Safety and tolerability, Progression Free survival (PFS), Overall Survival (OS), and Objective Response Rate (ORR) were assessed from the time of random assignment of those patients who continued to receive treatment at 1 year. The comparison was between a fixed 1-year treatment regimen and continuous therapy.
Of the 1,428 patients who received OPDIVO® in this study, 252 patients were randomly assigned to continuous treatment (N=127) or 1-year fixed-duration treatment (N=125). With minimum post-random assignment follow up of 13.5 months, median PFS was longer with continuous treatment versus 1-year fixed duration treatment (24.7 months versus 9.4 months; HR=0.56). Median Overall Survival from random assignment was also longer with continuous treatment versus 1-year fixed duration treatment in the Progression-Free Survival population (Not Reached versus 32.5 months; HR, 0.61), as well as in the Intent To Treat population (Not reached versus 28.8 months; HR, 0.62). New onset treatment-related Adverse Events occurred in a few patients and no new safety signals were identified.
The authors concluded that the above findings from an exploratory analysis represent the first randomized data on continuous versus fixed-duration immunotherapy, in previously treated patients with advanced NSCLC, and suggest that continuing OPDIVO® beyond 1 year improves outcomes.
Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non–Small-Cell Lung Cancer: CheckMate 153. Waterhouse DM, Garon EB, Chandler J, et al. DOI: 10.1200/JCO.20.00131 Journal of Clinical Oncology. Published online September 10, 2020.

