SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas.
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced NSCLC have a high level of PD-L1 expression and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®. The FDA approved KEYTRUDA® for the first-line treatment of advanced NSCLC with high PD-L1 expression (Tumor Proportion Score of 50% or more), and for previously treated advanced NSCLC with a PD-L1 Tumor Proportion Score of 1% or more. This present study provides convincing phase III evidence to support the use of triplet therapy in NSCLC.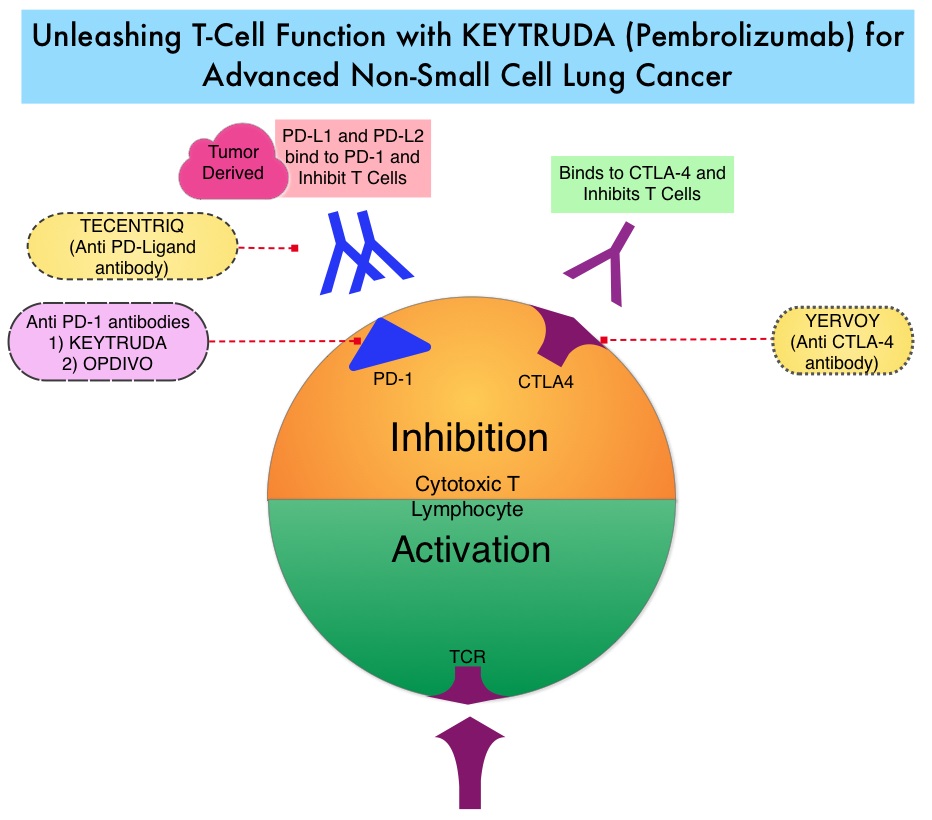
KEYNOTE-189 is a double-blind, phase III trial in which 616 patients with untreated stage IV non-squamous NSCLC, without sensitizing EGFR or ALK mutations, were randomly assigned in a 2:1 ratio to receive treatment with four cycles of KEYTRUDA®/Pemetrexed/Carboplatin or placebo plus the same chemotherapy. Patients received either KEYTRUDA® 200mg or saline placebo, both administered IV every 3 weeks for up to 35 cycles. All the patients received four cycles of the investigator’s choice of Cisplatin 75 mg/m2 IV or Carboplatin AUC 5 along with Pemetrexed 500 mg/m2, all administered IV every 3 weeks, followed by maintenance Pemetrexed 500 mg/m2 every 3 weeks. Patients in the placebo combination group were allowed to crossover to KEYTRUDA® monotherapy upon disease progression. Patients with symptomatic brain metastasis were excluded and patients were stratified according to PD-L1 expression (Tumor Proportion Score, 1% or more versus less than 1%), choice of platinum-based drug (Cisplatin versus Carboplatin), and smoking history. Both treatment groups were well balanced and about 17% had brain metastasis and one-third were untreated. A PD-L1 Tumor Proportion Score of 1% or more was reported in 63% of the patients, Carboplatin was the preferred platinum-based drug in 72% of the patients, and 88% of the patients were current or former smokers. The co-Primary end points were Overall Survival (OS) and Progression Free Survival (PFS).
After a median follow-up of 10.5 months, the estimated rate of Overall Survival (OS) at 12 months was 69.2% in the KEYTRUDA®-combination group versus 49.4% in the placebo-combination group. The median OS was not reached in the KEYTRUDA®-combination group and was 11.3 months in the placebo-combination group (HR=0.49; P<0.001). This represented a 51% reduction in the risk of death in the KEYTRUDA®-combination group. The OS benefit was seen across all PD-L1 subgroups including those with a PD-L1 Tumor Proportion Score of less than 1%. However, the greatest benefit was noted in the group expressing high levels of PD-L1 with Tumor Proportion Score 50% or more (12-month OS rate of 73% versus 48.1%; HR=0.42). The median Progression Free Survival was 8.8 months in the KEYTRUDA®-combination group and 4.9 months in the placebo-combination group (HR=0.52; P<0.001). This represented a 48% reduction in the risk of disease progression. The Objective Response Rate (ORR) was 47.6% in the KEYTRUDA®-combination group and 18.9% in the placebo-combination group (P<0.001) and ORR was highest among those with a PD-L1 Tumor Proportion Score of 50% or more (61.4% vs 22.9%). There was no significant difference in grade 3 adverse events in the two treatment groups.
It was concluded that in patients with previously untreated metastatic non-squamous NSCLC without EGFR or ALK mutations, the addition of KEYTRUDA® to standard chemotherapy of Pemetrexed and a platinum-based drug resulted in significantly longer Overall Survival and Progression Free Survival than chemotherapy alone, regardless of PD-L1 status and should therefore be considered as a new standard of care, in the front-line setting. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. for the KEYNOTE-189 Investigators. April 16, 2018 DOI: 10.1056/NEJMoa1801005

