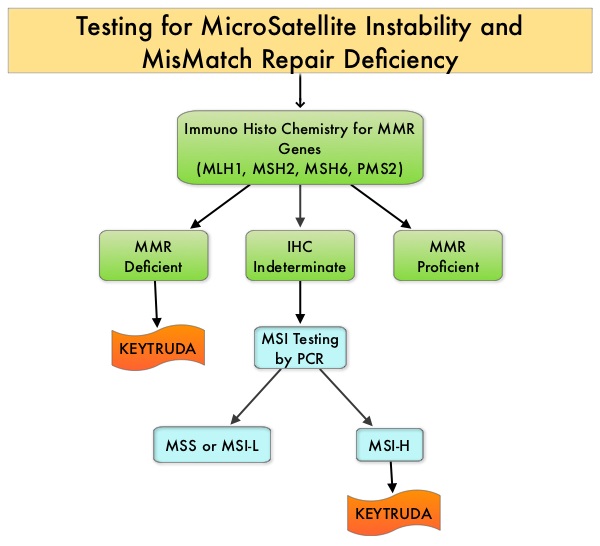
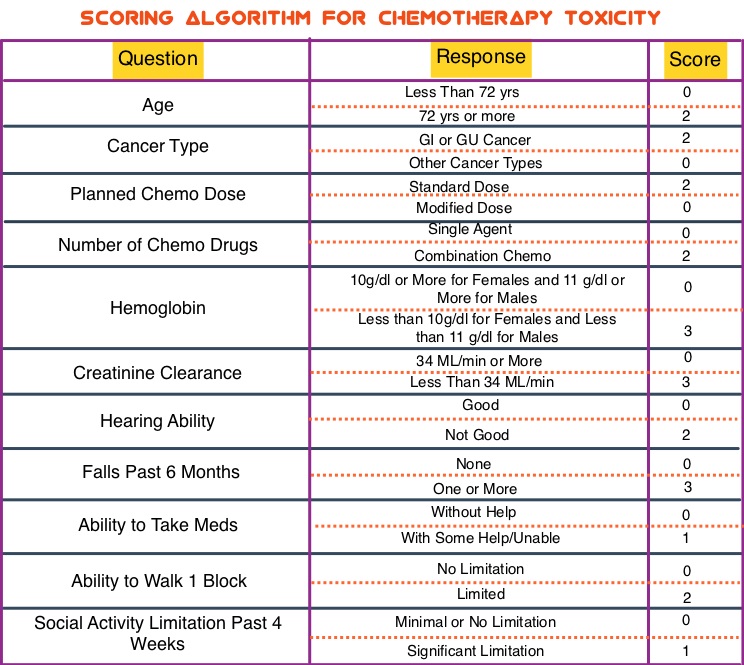
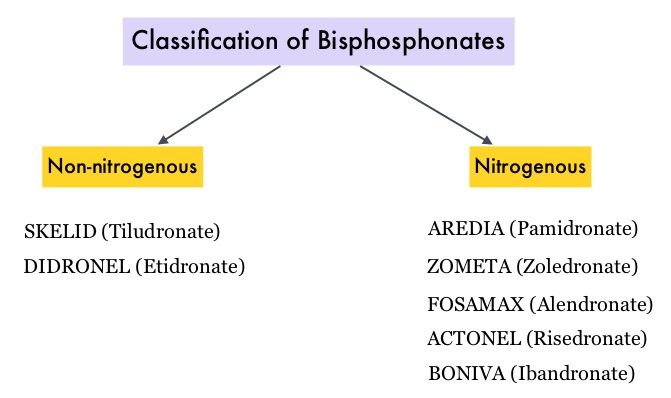
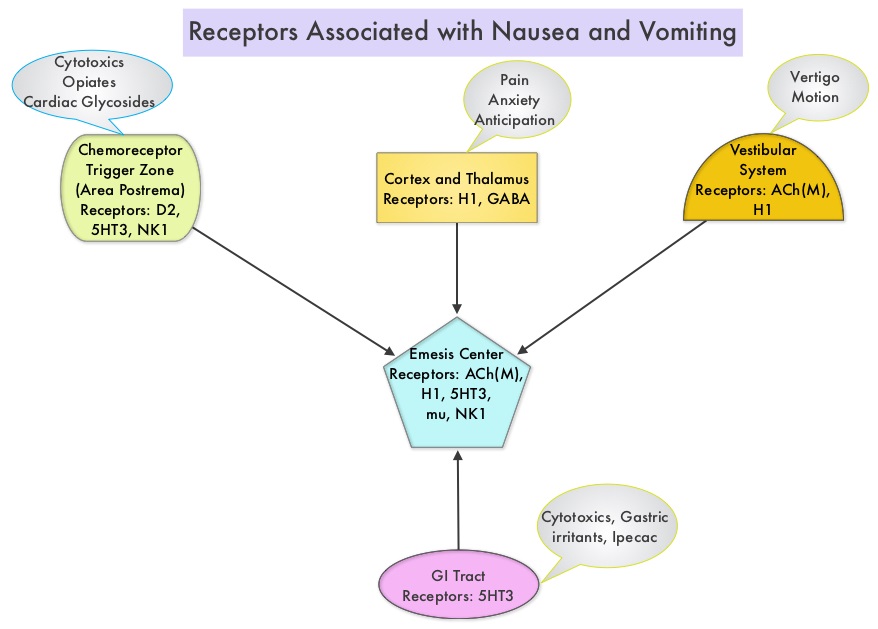
SUMMARY: Tumor genomic profiling enables the identification of specific genomic alterations and thereby can provide personalized treatment options with targeted therapies that are specific for those molecular targets. A genomic test can be performed on a tumor specimen or on cell-free DNA in plasma (“liquid biopsy”) or an ImmunoHistoChemistry (IHC) test can be performed on tumor tissue for protein expression that demonstrates a genomic variant known to be a drug target, or to predict sensitivity to a chemotherapeutic drug. Next-generation sequencing (NGS) platforms or second-generation sequencing unlike the first-generation sequencing, known as Sanger sequencing, perform massively parallel sequencing, which allows sequencing of millions of fragments of DNA from a single sample. With this high-throughput sequencing, the entire genome can be sequenced in less than 24 hours. Recently reported genomic profiling studies performed in patients with advanced cancer suggest that actionable mutations are found in 20-40% of patients’ tumors.
ProfiLER is an ongoing, molecular profiling clinical trial, developed to guide treatment by exploring genomic alterations in cancer cells of patients with advanced malignancy. DNA extracted from either archival or fresh tumor tissue was analyzed by next-generation sequencing of 60 cancer-related genes and whole-genome comparative genomic hybridization, a methodology for rapidly comparing DNA samples. A multidisciplinary board of experts in genomic profiling analyzed the genomic test results data and recommended molecular targeted therapies, when actionable mutations were found. These therapies were either commercially available drugs or those being tested in early clinical trials.
This study enrolled 2,676 patients to date and 1,944 tumors were analyzed. They included colorectal, gynecologic, breast, brain, and head and neck cancers, as well as sarcomas. Actionable mutations were found in 1,004 tumor samples (52%), 609 patients had only 1 actionable mutation, and 394 patients had 2-6 actionable mutations. The most common actionable mutation involved the PI3K/mTOR pathway. The molecular tumor board recommended molecularly targeted treatments to 676 patients (35% of 1,944 patients tested). Of these 676 patients, 143 received the recommended treatment, mostly through enrollment in a clinical trial. The rest of the 533 patients were unable to receive the recommended treatment because of poor health, rapid tumor progression, not meeting eligibility criteria for a clinical trial, or difficulty obtaining off-label commercially available drugs.
The Overall Survival rates for the 143 patients who received targeted therapies based on genomic testing was then compared with the 533 patients who did not. The Overall Survival rate at 3 years for those patients who received the recommended molecular targeted therapy was 53.7% compared with 46.1% for those patients who did not. The 5-year Overall Survival rate was also higher for patients who received molecular targeted therapy compared to those who did not (34.8% versus 28.1%).
This study validated that comprehensive genomic profiling can be performed in routine clinical practice, to select patients for targeted cancer therapies. The TAPUR (Targeted Agent and Profiling Utilization Registry) study conducted by ASCO is underway and is aimed at collecting “real-world” data on clinical outcomes, to help learn additional uses of molecularly-targeted cancer drugs, outside of indications approved by the FDA. Routine molecular screening of advanced refractory cancer patients: An analysis of the first 2490 patients of the ProfilER Study. Tredan O, Corset V, Wang Q, et al. J Clin Oncol 35, 2017 (suppl; abstr LBA100)