SUMMARY: The FDA on August 10, 2016 approved SUSTOL® (Granisetron) extended- release injection, for use in combination with other antiemetics in adults, for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of Moderately Emetogenic Chemotherapy (MEC) or Anthracycline and Cyclophosphamide (AC) combination chemotherapy regimen. Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment, whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on Serotonin (5-hydroxytryptamine-5HT3) and its receptors, with the chemotherapeutic agents stimulating the release of Serotonin from the enterochromaffin cells of the small intestine. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are also located centrally in the Chemoreceptor Trigger Zone of the area Postrema. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems.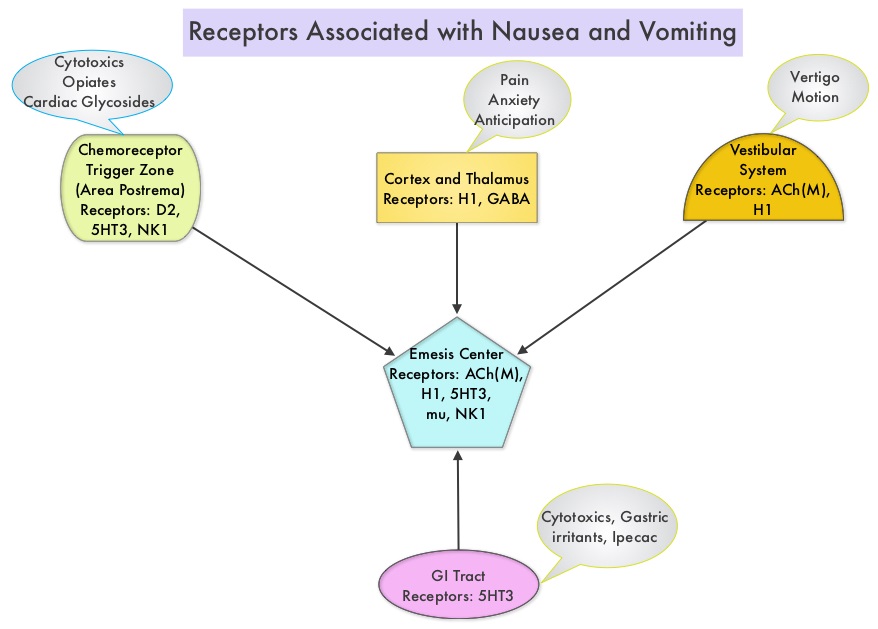
Granisetron is a first-generation serotonin-3 (5-HT3) receptor antagonist commonly used to treat CINV, but has a short half-life of approximately 8 hours. SUSTOL® is a novel formulation of 2 % Granisetron and a bioerodible tri(ethylene glycol) polymer, designed to provide slow, controlled hydrolysis, resulting in slow and sustained release of Granisetron, thereby preventing both acute and delayed CINV associated with MEC and Highly Emetogenic Chemotherapy (HEC). With a plasma half-life of 24-34 hours, this novel preparation formulation can maintain therapeutic levels of Granisetron for 5 or more days.
The FDA approval of SUSTOL® was based on the efficacy data from a multicenter double-blind trial which included 733 patients who received MEC or AC combination chemotherapy. Patients were randomized to receive either SUSTOL® 10 mg Subcutaneous injection (N=371) or ALOXI® 0.25 mg IV (N=362). This study was structured to compared SUSTOL® with ALOXI® (Palonosetron), which is also a serotonin-3 (5-HT3) receptor antagonist because, ALOXI® is not approved by the FDA to prevent delayed-onset CINV in patients who receive HEC. The trial was designed to allow for the evaluation of non-inferiority of SUSTOL® to ALOXI® in the acute and delayed phases. Antiemetics were administered 30 minutes before chemotherapy on Day 1. Dexamethasone was also administered IV at 8 or 20 mg on Day 1, depending on the chemotherapy regimen administered and those who received Dexamethasone 20 mg IV, were also given an 8 mg oral dose of the drug twice daily on Days 2, 3, and 4. The mean age was 57 years and 79% the patients were female. Approximately 55% of the patients received MEC regimen and 45% of the patients received AC combination chemotherapy. The most frequently used MEC regimen was Carboplatin and Paclitaxel combination (31%). The primary endpoint of the study was Complete Response (CR), which was defined as no emetic episodes or rescue medication during the acute phase (0-24 hours) and the delayed phase (more than 24 to 120 hours) after chemotherapy administration in cycle 1.
The results demonstrated that SUSTOL® was noninferior to ALOXI® . It was noted that among patients receiving MEC (N=406) the CR rate in the acute phase was 83% in the SUSTOL® group compared with 89% in the ALOXI® group. The CR rates in the delayed phase were 69% and 70% respectively. For those patients who received AC chemotherapy (N=327), the CR rate in the acute phase for the SUSTOL® group was 70% compared with 64% in the ALOXI® group. The CR rates in the delayed phase were 50% and 47% respectively. The safety of the FDA recommended dose of SUSTOL® (10 mg Subcutaneous dose), was evaluated in 924 patients across two double-blind, randomized active-controlled studies and it was noted that SUSTOL® was associated with a higher incidence of injection site reactions and constipation.
The authors concluded that SUSTOL® by virtue of its Biochronomer polymer-based drug delivery technology, represents a novel and convenient alternative to intravenous ALOXI® for the prevention of acute and delayed Chemotherapy Induced Nausea and Vomiting. Randomized phase III trial of APF530 versus palonosetron in the prevention of chemotherapy-induced nausea and vomiting in a subset of patients with breast cancer receiving moderately or highly emetogenic chemotherapy. Boccia R, O’Boyle E, Cooper W. BMC Cancer. 2016;16:166. doi:10.1186/s12885-016-2186-4.

