The FDA on November 6, 2017, granted regular approval to ZELBORAF® for the treatment of patients with Erdheim-Chester Disease (ECD) with BRAF V600 mutation. ZELBORAF® is a product of Hoffmann-La Roche Inc.
Tag: General Medical Oncology & Hematology
Alcohol and Cancer A Statement of the American Society of Clinical Oncology
SUMMARY: It has been estimated that in the United States, 3-4% of all cancer deaths are attributable to drinking alcohol. According to the Centers for Disease Control and Prevention, approximately 88,000 deaths were attributed to excessive alcohol use in the United States between 2006 and 2010. Alcohol consumption is an established risk factor for several malignancies, and is a potentially modifiable risk factor for cancer. The International Agency for Research on Cancer (IARC), a branch of WHO, classified alcohol as a group 1 carcinogen. The Cancer Prevention Committee of the American Society of Clinical Oncology has now provided an overview of the evidence of the links between alcohol drinking and cancer risk and cancer outcomes.
DRINKING GUIDELINES AND DEFINITIONS
The American Heart Association, American Cancer Society, and US Department of Health and Human Services all recommend that men limit intake to one to two drinks per day and women to one drink per day. People who do not currently drink alcohol should not start for any reason. A standard drink is defined as one that contains roughly 14 g of pure alcohol, which is the equivalent of 1.5 ounces of distilled spirits, 5 ounces of wine or 12 ounces of regular beer. Moderate drinking is defined at up to one drink per day for women and up to 2 drinks per day for men whereas heavy drinking is defined as 8 or more drinks per week or 3 or more drinks per day for women, and as many as 15 or more drinks per week or 4 or more drinks per day for men. Hispanics and blacks have a higher risk than whites, for developing alcohol-related liver disease. Use of alcohol during childhood and adolescence is a predictor of increased risk of alcohol related disorders later in life.
ROLE OF ALCOHOL IN CARCINOGENESIS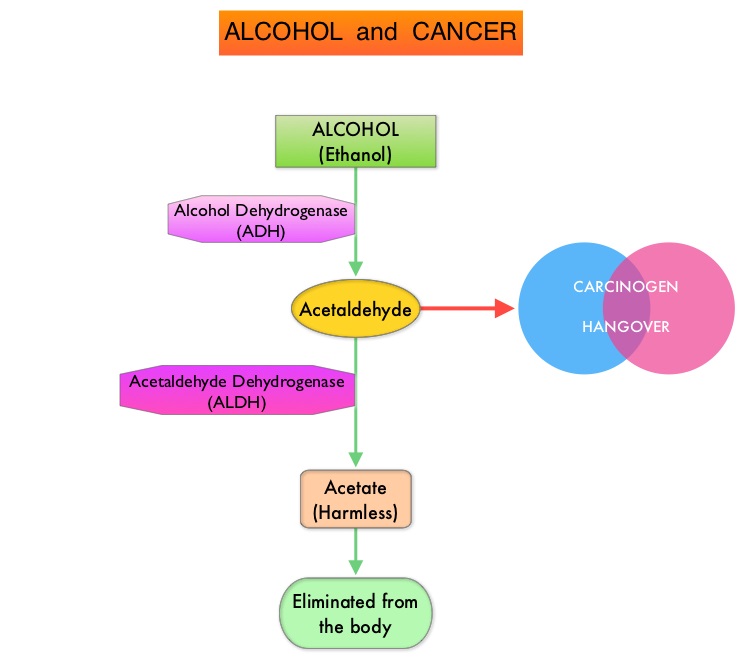
Alcohol is predominantly metabolized in the liver to acetaldehyde, which is a carcinogen and is responsible for many “hangover” symptoms. Acetaldehyde is then converted into harmless acetic acid radicals also known as acetyl radicals, and eliminated from the body. There is strong evidence to suggest that acetaldehyde damages DNA. Acetaldehyde generated during alcohol metabolism in the human body is eliminated by Aldehyde Dehydrogenase-2 (ALDH2). However, a genetic variant of ALDH2, which is an inactive form, exists and individuals with the inactive form of ALDH2 who consume alcohol, accumulate excessive amounts of acetaldehyde, which in turn can lead to greater susceptibility to alcohol-induced cancer. It has been noted that this high-risk genotype in prevalent in about 50% of North East Asian population and in 5–10% of blond-haired blue-eyed people of Northern European descent. Alcohol consumption in this group is more strongly associated with cancers of the upper aerodigestive tract. Breast tissue is also more susceptible to alcohol than other sites. Even moderate alcohol intake has been associated with increased levels of circulating sex hormones, which in turn can activate cellular proliferation. Alcohol consumption is associated with lower serum folate concentrations and this may play a role in the etiology of colon cancer.
ALCOHOL AND CANCER
There is a clear association between alcohol and upper aerodigestive tract cancers (larynx, esophagus, and oral cavity/pharynx), as a result of direct contact of ingested alcohol with the involved tissues.
Continued alcohol use among survivors of upper aerodigestive tract cancers is associated with a 3 fold increase in the risk of a second primary tumor in the upper aerodigestive tract. Additionally, there is a synergistic interaction between alcohol consumption and cigarette smoking. Smoking and alcohol use during and after radiation therapy have been associated with an increased risk of osteoradionecrosis of the jaw, in patients with oral and oropharyngeal cancers.
Among women with Estrogen Receptor-positive breast cancer, those consuming 7 or more drinks per week have a 90% increased risk of asynchronous contralateral breast cancer, versus those who do not consume alcohol. It is estimated that there is a 5% increase in premenopausal breast cancer per 10 grams of ethanol consumed per day and the risk is even greater at 9%, for postmenopausal breast cancer.
A recent meta-analysis of cohort studies among 209,597 cancer survivors showed an 8% increase in overall mortality and a 17% increased risk for recurrence in the highest versus lowest alcohol consumers and these numbers were statistically significant.
The benefit of alcohol consumption on cardiovascular health likely has been overstated and nondrinkers have lower rates of coronary heart disease and stroke than even light drinkers. Given the increase in the risk of cancer even with low levels of alcohol consumption, the net effect of alcohol is harmful. Alcohol consumption should therefore not be recommended to prevent cardiovascular disease or all-cause mortality.
In conclusion, alcohol is a well-established risk factor for the development of certain cancers and further research is needed to understand the effects of alcohol exposure on the efficacy of chemotherapy, immunotherapy and radiation treatment. Alcohol and Cancer: A Statement of the American Society of Clinical Oncology. LoConte NK, Brewster AM, Kaur JS, et al. DOI: 10.1200/JCO.2017.76.1155 Journal of Clinical Oncology – published online before print November 7, 2017
FDA Approves First Biosimilar for Cancer Treatment
SUMMARY: The FDA on Sept. 14, 2017 approved MVASI® (Bevacizumab-awwb) as a Biosimilar to AVASTIN® (Bevacizumab). Bevacizumab is a recombinant immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that binds to Vascular Endothelial Growth Factor (VEGF) and inhibits the interaction of VEGF with its receptors, VEGF receptor-1 and VEGF receptor-2. This in turn inhibits establishment of new blood vessels that are essential for the maintenance and growth of solid tumors. MVASI® is the first Biosimilar approved in the U.S. for the treatment of cancer. A Biosimilar product is a biological product that is approved based on its high similarity to an already approved biological product (also known as reference product). Biological products are made from living organisms including humans, animals and microorganisms such as bacteria or yeast, and are manufactured through biotechnology, derived from natural sources or produced synthetically. Biological products have larger molecules with a complex structure, than conventional drugs (also known as small molecule drugs).
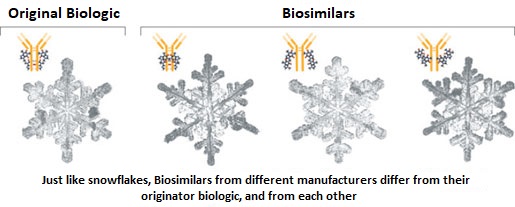 Unlike biological products, conventional drugs are made of pure chemical substances and their structures can be identified. A generic drug is a copy of brand name drug and has the same active ingredient and is the same as brand name drug in dosage form, safety and strength, route of administration, quality, performance characteristics and intended use. Therefore, brand name and the generic drugs are bioequivalent. The Affordable Care Act in 2010 created an abbreviated licensure pathway for biological products that are demonstrated to be “Biosimilar” to, or “interchangeable” with an FDA approved biological product (reference product). The Biosimilar must show that it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. A Biosimilar product can only be approved by the FDA if it has the same mechanism of action, route of administration, dosage form and strength as the reference product, and only for the indications and conditions of use that have been approved for the reference product. Biosimilars are not as easy to manufacture as generics (copies of brand name drugs), because of the complexity of the structure of the biologic product and the process used to make a biologic product. The facilities where Biosimilars are manufactured must also meet the FDA standards.
Unlike biological products, conventional drugs are made of pure chemical substances and their structures can be identified. A generic drug is a copy of brand name drug and has the same active ingredient and is the same as brand name drug in dosage form, safety and strength, route of administration, quality, performance characteristics and intended use. Therefore, brand name and the generic drugs are bioequivalent. The Affordable Care Act in 2010 created an abbreviated licensure pathway for biological products that are demonstrated to be “Biosimilar” to, or “interchangeable” with an FDA approved biological product (reference product). The Biosimilar must show that it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. A Biosimilar product can only be approved by the FDA if it has the same mechanism of action, route of administration, dosage form and strength as the reference product, and only for the indications and conditions of use that have been approved for the reference product. Biosimilars are not as easy to manufacture as generics (copies of brand name drugs), because of the complexity of the structure of the biologic product and the process used to make a biologic product. The facilities where Biosimilars are manufactured must also meet the FDA standards.
MVASI® is approved for the treatment of patients with the following cancers:
• Metastatic Colorectal cancer, in combination with intravenous 5-Fluorouracil-based chemotherapy for first or second line treatment. MVASI® is not indicated for the adjuvant treatment of surgically resected Colorectal cancer.
• Metastatic Colorectal cancer, in combination with Fluoropyrimidine-Irinotecan or Fluoropyrmidine-Oxaliplatin-based chemotherapy for the second line treatment of patients who have progressed on a first-line Bevacizumab containing regimen.
• Non-squamous Non Small Cell Lung Cancer, in combination with Carboplatin and Paclitaxel for first line treatment of unresectable, locally advanced, recurrent or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in Objective Response Rate. No data is available demonstrating improvement in disease-related symptoms or survival with Bevacizumab.
• Metastatic Renal cell carcinoma, in combination with Interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic disease, in combination with Paclitaxel and Cisplatin or Paclitaxel and Topotecan.
The approval of MVASI® was based on two studies. In the first study, PharmacoKinetics (PK) of biosimilar MVASI® was compared with Bevacizumab, following a single infusion of 3 mg/kg. It was concluded that the PK data was similar between the Biosimilar, MVASI® and Bevacizumab. The second study is a randomized, double-blind, phase III trial, that evaluated the efficacy and safety of MVASI®, compared with Bevacizumab, in patients with non-squamous Non Small Cell Lung Cancer (NSCLC). Patients with non-squamous NSCLC, on first line chemotherapy with Carboplatin and TAXOL® (Paclitaxel), were randomized in a 1:1 ratio to receive either MVASI® (N=328) or Bevacizumab 15 mg/kg (N=314), as an IV infusion, every 3 weeks, for 6 cycles. The Objective Response Rate (ORR) was similar between the two treatment groups (39.0% for MVASI® and 41.7% for Bevacizumab) and these results were not statistically different. The Duration of Response was similar. Adverse events were comparable in the two treatment groups. This study demonstrated that MVASI® was clinically similar to Bevacizumab.
The FDA concluded that the approval of MVASI® was based on comparisons of extensive structural and functional product characterization, animal data, human PharmacoKinetic and pharmacodynamic data, clinical immunogenicity, between MVASI® and AVASTIN® (Bevacizumab), and it was noted that MVASI® is highly similar to AVASTIN® and that there are no clinically meaningful differences between the two products. Randomized, double-blind, phase 3 study evaluating efficacy and safety of ABP 215 compared with bevacizumab in patients with non-squamous NSCLC. Thatcher N, Thomas M, Paz-Ares L, et al. DOI: 10.1200/JCO.2016.34.15_suppl.9095 Journal of Clinical Oncology 34, no. 15_suppl (May 2016) 9095-9095.
MVASI® (Bevacizumab-awwb)
The FDA on September 14, 2017 approved MVASI® as a biosimilar to AVASTIN® (Bevacizumab). MVASI® is the first biosimilar approved in the U.S. for the treatment of cancer, and is a product of Amgen Inc.
FDA Approves First Biosimilar for Cancer Treatment
The FDA on Sept. 14, 2017 approved MYASI® (Bevacizumab-awwb) as a Biosimilar to AVASTIN® (Bevacizumab). MYASI® is the first Biosimilar approved in the U.S. for the treatment of cancer. A Biosimilar must show that it has no clinically meaningful differences in terms of safety and effectiveness from the already approved biological product (also known as reference product). A Biosimilar product can only be approved by the FDA if it has the same mechanism of action, route of administration, dosage form and strength as the reference product, and only for the indications and conditions of use, that have been approved for the reference product. The approval of MYASI® was based on comparisons of extensive structural and functional product characterization, animal data, human PharmacoKinetic and pharmacodynamic data, clinical immunogenicity, between MYASI® and AVASTIN® (Bevacizumab), and it was noted that MYASI® is highly similar to AVASTIN® and that there are no clinically meaningful differences between the two products.
Late Breaking Abstract – ASCO 2017 Single Dose Radiation Therapy as Effective as Multiple Fractions for Metastatic Spinal Cord Compression
SUMMARY: Metastatic Spinal Cord Compression (MSCC) first described by Spiller in 1925, is an oncologic emergency and is a well recognized complication of cancer. Approximately 10% of all patients with cancer develop metastatic disease to the spinal column. Even though any solid tumor can metastasize to the spine, more than 50% of MSCC cases are caused by breast cancer, prostate cancer and lung cancer. The risk of MSCC is particularly high in those patients with widespread malignancy and those with known spinal metastases. Pathological compression fracture of the vertebral body or direct tumor invasion can cause compression of the spinal cord or cauda equina resulting in irreversible neurological deficit as well as paraplegia. Common symptoms include back pain, tingling, numbness and difficulty walking. Early recognition of symptoms and prompt intervention is therefore imperative to prevent neurological damage.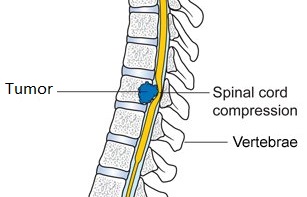
Patients with MSCC, in addition to steroids, are often treated with Radiation Therapy (RT) to relieve pain and improve neurological function and mobility. There is however no standard Radiation Therapy schedule. ASTRO (American Society for Radiation Oncology) in its “Choosing Wisely” guidelines recommended not using extended fractionated schemes (more than 10 fractions) for palliation of bone metastases. Equivalent pain relief can be accomplished following 30 Gy in 10 fractions, 20 Gy in 5 fractions, or a single 8 Gy fraction. It was also recommended that strong consideration should be given to a single 8 Gy fraction, for patients with a limited prognosis or with transportation difficulties.
SCORAD III is a randomized phase III study which evaluated whether a single-dose Radiation Therapy (RT) was as effective as multifraction RT administered over 5 days, without compromising patient outcomes. Enrolled patients (N=688) were randomized 1:1 to receive External Beam spinal canal RT as a single dose of 8 Gy (N=345) or 20 Gy in 5 fractions (N=343). Eligible patients had spinal cord or cauda equina (C1-S2) compression, confirmed by MRI/CT scan, treatable within a single radiation field, with no prior RT to the same area and had a life expectancy of more than 8 weeks. The median age was 70 years, 73% were male and 44% had metastatic prostate, 18% had metastatic lung, 11% had metastatic breast and another 11% had metastatic GastroIntestinal cancers. Patients were stratified by Ambulatory Status (AS), site of primary, and presence or absence of non-skeletal metastases. The primary endpoint of the study was Ambulatory Status, measured on a four-point scale – Grade 1: Able to walk normally, Grade 2: Able to walk with a walking aid (such as a cane or walker), Grade 3: Has difficulty walking even with walking aids and Grade 4: Dependent on wheelchair. Two third of the patients (66%) were ambulatory with or without walking aids (Ambulatory Status of 1 to 2) at study entry.
It was noted that at 8 weeks, 69.5% of patients who received single-dose radiation therapy and 73.3% of those who received five doses had an Ambulatory Status of 1 to 2 and could walk normally or with a walking aid such as a cane or a walker, suggesting that both single dose and longer course radiation treatments helped patients with their mobility. The median Overall Survival was similar in the two treatment groups – 12.4 weeks with a single dose versus 13.7 weeks with five doses, and this was not statistically significant (HR=1.02; P=0.81). The proportion of patients experiencing severe side effects was similar in the two treatment groups (20.6% vs 20.4%), but mild side effects were less common in the single dose of 8 Gy group compared to those receiving multiple fractions (51% vs 56.9%).
The authors concluded that a single radiation dose of 8 Gy in patients with metastatic Spinal Cord Compression was non-inferior and was as effective as longer course multiple fractions, for Ambulatory Status at 8 weeks, as well as Overall Survival. They added that this would mean fewer hospital visits and more time with the family, at least for patients with a short life expectancy. It should however be noted that in this study, at the time of enrollment, majority of patients were fully ambulatory or were able to walk with a walking aid. Whether single dose radiation therapy is adequate for those patients with very advanced involvement of the spine, however remains to be seen. SCORAD III: Randomized non-inferiority phase III trial of single dose radiotherapy (RT) compared to multifraction RT in patients (pts) with metastatic spinal canal compression (SCC). Hoskin P, Misra V, Hopkins K, et al. J Clin Oncol. 35;2017 (suppl; abstr LBA10004).
IMBRUVICA® (Ibrutinib)
The FDA on August 2, 2017 approved IMBRUVICA® for the treatment of adult patients with chronic Graft Versus Host Disease (cGVHD), after failure of one or more lines of systemic therapy. This is the first FDA-approved therapy for the treatment of cGVHD. IMBRUVICA® is a product of Pharmacyclics LLC.
Antiemetics American Society of Clinical Oncology Clinical Practice Guideline Update
SUMMARY: The ASCO guideline for Antiemetics in oncology was updated by the ASCO Expert Panel following a systematic review of 41publications from November 2009 thru June 2016. The recommendations in this guideline are most definitive for adults who are treated with single-day IV chemotherapy. This topic has been divided into Part I and Part II for easy reading. Part II is continued in the second article of this e NewsLetter.
Guideline Question: What are the most effective strategies for preventing or managing nausea and vomiting due to antineoplastic agents or radiation therapy?
Target Population: Adults and children who receive antineoplastic agents and adults who undergo radiation therapy for cancer.
Target Audience: Medical and Radiation Oncologists, Oncology Nurses, Nurse Practitioners, Physician Assistants, Oncology Pharmacists, and Patients with cancer
KEY RECOMMENDATIONS – PART I
Adult Patients
High-emetic-risk antineoplastic agents
• (Updated) Adult patients who are treated with Cisplatin and other high-emetic-risk single agents should be offered a four-drug combination of a Neurokinin 1 (NK1) receptor antagonist, a Serotonin (5-HT3) receptor antagonist, Dexamethasone, and Olanzapine. Dexamethasone and Olanzapine should be continued on days 2 to 4.
• (Updated) Adult patients who are treated with an Anthracycline combined with Cyclophosphamide should be offered a four-drug combination of an NK1 receptor antagonist, a 5-HT3 receptor antagonist, Dexamethasone, and Olanzapine. Olanzapine should be continued on days 2 to 4.
Moderate-emetic-risk antineoplastic agents
• (Updated) Adult patients who are treated with Carboplatin AUC 4 or more should be offered a three-drug combination of an NK1 receptor antagonist, a 5-HT3 receptor antagonist, and Dexamethasone.
• (Updated) Adult patients who are treated with moderate-emetic-risk antineoplastic agents, excluding Carboplatin AUC 4 or more, should be offered a two-drug combination of a 5-HT3 receptor antagonist (day 1) and Dexamethasone (day 1).
• (Updated) Adult patients who are treated with Cyclophosphamide, Doxorubicin, Oxaliplatin, and other moderate-emetic-risk antineoplastic agents that are known to cause delayed nausea and vomiting may be offered Dexamethasone on days 2 to 3.
Low-emetic-risk antineoplastic agents
• (Updated) Adult patients who are treated with low-emetic-risk antineoplastic agents should be offered a single dose of a 5-HT3 receptor antagonist or a single 8-mg dose of Dexamethasone before antineoplastic treatment.
Minimal-emetic-risk antineoplastic agents
• (Reworded for clarity) Adult patients who are treated with minimal-emetic-risk antineoplastic agents should not be offered routine antiemetic prophylaxis.
Antineoplastic combinations
• (Reworded for clarity) Adult patients who are treated with antineoplastic combinations should be offered antiemetics that are appropriate for the component antineoplastic agent of greatest emetic risk.
Adjunctive drugs
• (Updated) Lorazepam is a useful adjunct to antiemetic drugs, but is not recommended as a single-agent antiemetic.
Cannabinoids
• (New) Evidence remains insufficient for a recommendation regarding treatment with medical marijuana for the prevention of nausea and vomiting in patients with cancer who receive chemotherapy or radiation therapy. Evidence is also insufficient for a recommendation regarding the use of medical marijuana in place of the tested and US FDA-approved cannabinoids, Dronabinol and Nabilone, for the treatment of nausea and vomiting caused by chemotherapy or radiation therapy.
Complementary and alternative therapies
• (Reworded for clarity) Evidence remains insufficient for a recommendation for or against the use of ginger, acupuncture/acupressure, and other complementary or alternative therapies for the prevention of nausea and vomiting in patients with cancer.
High-dose chemotherapy with stem cell or bone marrow transplantation
• (Updated) Adult patients who are treated with high-dose chemotherapy and stem cell or bone marrow transplantation should be offered a three-drug combination of an NK1 receptor antagonist, a 5-HT3 receptor antagonist, and Dexamethasone.
Multiday antineoplastic therapy
• (Reworded for clarity) Adult patients who are treated with multiday antineoplastic agents should be offered antiemetics before treatment that are appropriate for the emetic risk of the antineoplastic agent administered on each day of the antineoplastic treatment and for 2 days after the completion of the antineoplastic regimen.
• (Strengthened) Adult patients who are treated with 4- or 5-day Cisplatin regimens should be offered a three-drug combination of an NK1 receptor antagonist, a 5-HT3 receptor antagonist, and Dexamethasone.
Continued….. in Article 2 of this e NewsLetter
Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. Hesketh PJ, Kris MG, Basch E, et al. DOI: 10.1200/JCO.2017.74.4789 Journal of Clinical Oncology – published online before print July 31, 2017
Antiemetics American Society of Clinical Oncology Clinical Practice Guideline Update (Part II)
SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is quite common and occurs in about 80% of patients receiving chemotherapy. The following (Part II) is a continuation of the ASCO Antiemetics Clinical Practice Guideline Update.
KEY RECOMMENDATIONS (ctd) – PART II
Adult Patients
Breakthrough nausea and vomiting
• (No change) For patients with breakthrough nausea or vomiting, clinicians should re-evaluate emetic risk, disease status, concurrent illnesses, and medications, and ascertain that the best regimen is being administered for the emetic risk.
• (Updated) Adult patients who experience nausea or vomiting despite optimal prophylaxis, and who did not receive Olanzapine prophylactically, should be offered Olanzapine in addition to continuing the standard antiemetic regimen.
• (Updated) Adult patients who experience nausea or vomiting despite optimal prophylaxis, and who have already received Olanzapine, may be offered a drug of a different class—for example, an NK1 receptor antagonist, Lorazepam or Alprazolam, a dopamine receptor antagonist, Dronabinol, or Nabilone—in addition to continuing the standard antiemetic regimen.
Anticipatory nausea and vomiting
• (Reworded for clarity) All patients should receive the most active antiemetic regimen that is appropriate for the antineoplastic agents being administered. Clinicians should use such regimens with initial antineoplastic treatment, rather than assessing the patient’s emetic response with less effective antiemetic treatment. If a patient experiences anticipatory emesis, clinicians may offer behavioral therapy with systematic desensitization.
KEY RECOMMENDATIONS
High emetic risk Radiation Therapy
• (Updated) Adult patients who are treated with high-emetic-risk radiation therapy should be offered a two-drug combination of a 5-HT3 receptor antagonist and Dexamethasone before each fraction and on the day after each fraction if Radiation Therapy is not planned for that day.
Moderate-emetic-risk radiation therapy
• (Reworded for clarity) Adult patients who are treated with moderate-emetic-risk Radiation Therapy should be offered a 5-HT3 receptor antagonist before each fraction, with or without Dexamethasone before the first five fractions. Low-emetic-risk radiation therapy
• (Updated) Adult patients who are treated with Radiation Therapy to the brain should be offered rescue Dexamethasone therapy. Patients who are treated with Radiation Therapy to the head and neck, thorax, or pelvis should be offered rescue therapy with a 5-HT3 receptor antagonist, Dexamethasone, or a Dopamine receptor antagonist.
Minimal-emetic-risk radiation therapy
• (Updated) Adult patients who are treated with minimal-emetic-risk radiation therapy should be offered rescue therapy with a 5-HT3 receptor antagonist, Dexamethasone, or a Dopamine receptor antagonist.
Concurrent radiation and antineoplastic agent therapy
• (Updated) Adult patients who are treated with concurrent radiation and antineoplastic agents should receive antiemetic therapy that is appropriate for the emetic risk level of antineoplastic agents, unless the risk level of the radiation therapy is higher. During periods when prophylactic antiemetic therapy for antineoplastic agents has ended and ongoing radiation therapy would normally be managed with its own prophylactic therapy, patients should receive prophylactic therapy that is appropriate for the emetic risk of the radiation therapy until the next period of antineoplastic therapy, rather than receiving rescue therapy for antineoplastic agents as needed.
Pediatric Patients
High-emetic-risk antineoplastic agents
• (Updated) Pediatric patients who are treated with high-emetic-risk antineoplastic agents should be offered a three-drug combination of a 5-HT3receptor antagonist, Dexamethasone, and Aprepitant.
• (New) Pediatric patients who are treated with high-emetic-risk antineoplastic agents who are unable to receive Aprepitant should be offered a two-drug combination of a 5-HT3 receptor antagonist and Dexamethasone.
• (New) Pediatric patients who are treated with high-emetic-risk antineoplastic agents who are unable to receive Dexamethasone should be offered a two-drug combination of Palonosetron and Aprepitant.
Moderate-emetic-risk antineoplastic agents
• (Reworded for clarity) Pediatric patients who are treated with moderate-emetic-risk antineoplastic agents should be offered a two-drug combination of a 5-HT3receptor antagonist and Dexamethasone.
• (New) Pediatric patients who are treated with moderate-emetic-risk antineoplastic agents who are unable to receive Dexamethasone should be offered a two-drug combination of a 5-HT3 receptor antagonist and Aprepitant.
Low-emetic-risk antineoplastic agents
• (New) Pediatric patients who are treated with low-emetic-risk antineoplastic agents should be offered Ondansetron or Granisetron.
Minimal emetic risk antineoplastic agents
• (New) Pediatric patients who are treated with minimal-emetic-risk antineoplastic agents should not be offered routine antiemetic prophylaxis.
Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. Hesketh PJ, Kris MG, Basch E, et al. DOI: 10.1200/JCO.2017.74.4789 Journal of Clinical Oncology – published online before print July 31, 2017
FDA’s First Tissue/Site-Agnostic Approval
The FDA for the first time approved a cancer treatment based on specific genetic biomarker, rather than location in the body where the tumor originated. KEYTRUDA®, an anti-PD1 monoclonal antibody was granted accelerated approval for treatment of adult and pediatric patients with unresectable or metastatic, MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options or with MSI-H or dMMR ColoRectal Cancer that has progressed following treatment with a Fluoropyrimidine, Oxaliplatin, and Irinotecan. MMR gene deficiency can be detected by ImmunoHistoChemistry and MSI testing is performed using a PCR based assay.
