The FDA on June 7, 2018 approved MIRCERA® for the treatment of pediatric patients 5 to 17 years of age on hemodialysis who are converting from another ESA after their hemoglobin level was stabilized with an ESA. MIRCERA® is a product of Vifor Pharma Inc.
Tag: General Medical Oncology & Hematology
FULPHILA® (Pegfilgrastim-jmdb)
The FDA on June 4, 2018 approved FULPHILA® as a biosimilar to NEULASTA® (Pegfilgrastim, Amgen, Inc.), to decrease the chance of infection as suggested by febrile neutropenia in patients with non-myeloid cancer, who are receiving myelosuppressive chemotherapy that has a clinically significant incidence of febrile neutropenia. FULPHILA® is a product of Mylan GmbH.
DOPTELET® (Avatrombopag)
The FDA on May 21, 2018 approved DOPTELET® for thrombocytopenia in adults with chronic liver disease scheduled to undergo a procedure. DOPTELET® is a product of AkaRx Inc.
FDA Approves 4-Week Dosing Schedule for OPDIVO®
SUMMARY: The FDA on March 6, 2018 approved a supplemental Biologics License Application (sBLA) updating the OPDIVO® (Nivolumab) dosing schedule to include 480 mg infused every four weeks (Q4W) for a majority of approved indications. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody approved by the FDA for multiple tumor types. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Under normal circumstances, Immune checkpoints or gate keepers, inhibit intense immune responses by switching off the T cells of the immune system. They therefore suppress antitumor immunity. OPDIVO® by targeting immune checkpoint PD-1, unleashes the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The clinical pharmacology of OPDIVO® is well established and the clinical data regarding the efficacy and safety of OPDIVO® when administered at 3 mg/kg Q2W (every 2 weeks) across multiple tumor types is well characterized. However, alternative dosing schedules would provide flexibility and other benefits both to patients as well as prescribers.
The authors in this study, using a combination of quantitative clinical pharmacology analyses and safety assessments, evaluated the feasibility of extending the dosing interval of OPDIVO®, and administering it every 4 weeks instead of every 2 weeks. They examined the predicted risk/benefit profile of OPDIVO® 480 mg Q4W compared to 3 mg/kg Q2W by
(1) Comparing OPDIVO® exposures produced by 3 mg/kg Q2W and 480 mg Q4W across tumor types
(2) Evaluating OPDIVO® exposure margins for safety, relative to the well-tolerated dose of 10 mg/kg Q2W
(3) Comparing the predicted risk of experiencing grade 3 adverse events with 480 mg Q4W relative to 3 mg/kg Q2W across the various tumor types for which it is indicated
(4) Comparing the predicted Objective Response Rate (ORR) and Overall Survival with OPDIVO® 480 mg Q4W compared to 3 mg/kg Q2W, in patients with Melanoma, Non Small Cell Lung Cancer (NSCLC), and Renal Cell Carcinoma (RCC).
The researchers noted that among patients with Melanoma, NSCLC, or RCC, there was a less than 1% difference in the predicted probability of achieving a response. The predicted 1 and 2-year survival probabilities were also similar among patients with these tumor types receiving either of the two dose schedules of OPDIVO®, with differences ranging between 0-4.6% at the end of the first year and 1.9-6.9% at the end of second year, across tumor types.
Based on this data, OPDIVO® 480 mg Q4W flat dose option was approved by the FDA for the following indications:
• Metastatic melanoma (monotherapy or monotherapy phase after combination treatment with YERVOY® (Ipilimumab)
• Previously treated metastatic Non Small Cell Lung Cancer
• Advanced Renal Cell Carcinoma following prior Anti-angiogenic therapy
• Previously treated locally advanced or metastatic Urothelial carcinoma following disease progression during or after Platinum-based chemotherapy
• Classical Hodgkin lymphoma following relapse/progression after autologous Hematopoietic Stem Cell Transplantation (HSCT) and Brentuximab vedotin, or three or more lines of systemic therapy that includes autologous HSCT
• Recurrent/metastatic Squamous Cell Carcinoma of the Head and Neck following Platinum-based therapy
• Hepatocellular carcinoma after prior Sorafenib therapy
• Adjuvant therapy for patients with completely resected Melanoma with lymph node involvement or metastatic disease
It was concluded that based on the clinical pharmacology of OPDIVO® and well characterized Exposure-Response relationships for efficacy and safety, the differences in exposures produced by a OPDIVO® schedule of 480 mg Q4W relative to 3 mg/kg Q2W dosing schedule, should not result in clinically meaningful differences in the safety and efficacy of OPDIVO®. This alternate, flexible dosing option may further help tailor patient care. A model-based exposure-response (E-R) assessment of a nivolumab (NIVO) 4-weekly (Q4W) dosing schedule across multiple tumor types [abstract]. Zhao X, Ivaturi V, Gopalakrishnan M, et al. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017;77(13 Suppl):Abstract CT101. doi:10.1158/1538-7445.AM2017-CT101
Larotrectinib – A Novel Age and Tumor Agnostic Therapy for TRK Fusion-Positive Cancers
Tumor genomic profiling enables the identification of specific genomic alterations and thereby can provide personalized treatment options with targeted therapies that are specific for those molecular targets. Next-Generation Sequencing (NGS) platforms or second-generation sequencing perform massively parallel sequencing, which allows sequencing of millions of fragments of DNA from a single sample. Recently reported genomic profiling studies performed in patients with advanced cancer suggest that actionable mutations are found in 20-40% of patients’ tumors.
Larotrectinib is a potent and highly selective small molecule inhibitor of three TRK proteins, Tropomyosin Receptor Kinase genes NTRK1, NTRK2, and NTRK3. In a phase I-II study involving children, adolescents and adults with 17 unique cancer diagnoses, the Overall Response Rate was 75% and at 1 year, 71% of the responses were ongoing and 55% of the patients remained progression-free. It was concluded from this study that TRK fusions defined a unique molecular subgroup of advanced solid tumors in children and adults and Larotrectinib had marked and durable antitumor activity in patients with TRK fusion-positive cancer, regardless of the age of the patient or tumor type.
Larotrectinib – A Novel Age and Tumor Agnostic Therapy for TRK Fusion-Positive Cancers
SUMMARY: Tumor genomic profiling enables the identification of specific genomic alterations and thereby can provide personalized treatment options with targeted therapies that are specific for those molecular targets. The FDA in May 2017, granted accelerated approval to KEYTRUDA® (Pembrolizumab), for adult and pediatric patients with unresectable or metastatic, MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options. This is the first FDA approval of a systemic cancer treatment, based on a specific genetic biomarker, independent of tumor origin (first tissue/site-agnostic approval).
A genomic test can be performed on a tumor specimen or on cell-free DNA in plasma (“liquid biopsy”) or an ImmunoHistoChemistry (IHC) test can be performed on tumor tissue for protein expression that demonstrates a genomic variant known to be a drug target, or to predict sensitivity to a chemotherapeutic drug. Next-generation sequencing (NGS) platforms or second-generation sequencing unlike the first-generation sequencing, known as Sanger sequencing, perform massively parallel sequencing, which allows sequencing of millions of fragments of DNA from a single sample. With this high-throughput sequencing, the entire genome can be sequenced in less than 24 hours. Recently reported genomic profiling studies performed in patients with advanced cancer suggest that actionable mutations are found in 20-40% of patients’ tumors.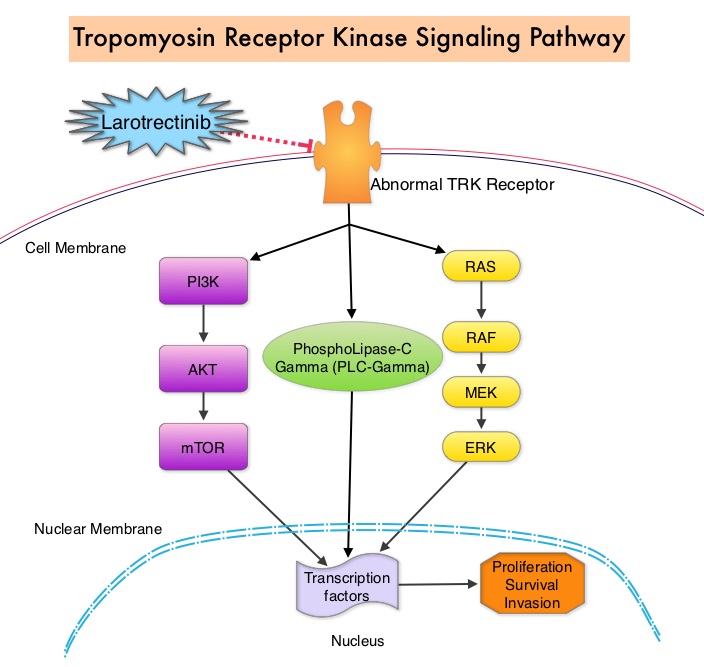
The molecules of interest are Neurotrophic Tropomyosin Receptor Kinase (NTRK) gene rearrangements. The three TRK family of Tropomyosin Receptor Kinase (TRK) transmembrane proteins TRKA, TRKB, and TRKC are encoded by Neurotrophic Tropomyosin Receptor Kinase genes NTRK1, NTRK2, and NTRK3, respectively. These receptor tyrosine kinases are expressed in human neuronal tissue and are involved in a variety of signaling events such as cell differentiation, cell survival and apoptosis of peripheral and central neurons. They therefore play an essential role in the physiology of development and function of the nervous system. Chromosomal fusion involving NTRK genes represent the main molecular alterations with known oncogenic and transforming potential and have been identified in a variety of cancers both in children and adults. Gene fusions involving NTRK genes lead to transcription of chimeric TRK proteins which can confer oncogenic potential by increasing cell proliferation and survival. These genetic abnormalities have generated a lot of interest and have emerged as targets for cancer therapy. NGS has allowed the discovery of these gene fusions. Early clinical evidence suggests that these gene fusions lead to oncogene addiction regardless of tissue of origin. (Oncogene addiction is the dependency of some cancers on one or a few genes for the maintenance of the malignant phenotype).
Larotrectinib is a potent and highly selective small molecule inhibitor of all three TRK proteins. The authors in this development program included patients of any age and with any tumor type who had chromosomal fusion involving NTRK genes (Age and Tumor agnostic therapy). This program enrolled 55 patients, ranging in age from 4 months to 76 years, with consecutively and prospectively identified TRK fusion-positive cancers, detected by molecular profiling. They were assigned to three clinical studies – a phase I study involving adults, a phase I-II study involving children and a phase II study involving adolescents and adults.
This population of patients encompassed 17 unique cancer diagnoses and enrolled patients had locally advanced or metastatic solid tumors, and had received prior standard therapy (if available). The Primary end point for the combined analysis was the Overall Response Rate according to Independent review. Secondary end points included Duration of Response, Progression Free Survival, and safety. The researchers in this publication reported the safety and efficacy analysis of the first 55 consecutively enrolled patients, identified with TRK fusion-positive cancers, treated across these studies.
The Overall Response Rate was 75%. A total of 13% of the patients had a Complete Response, 62% had a Partial Response and 13% had stable disease. The median Duration of Response and Progression Free Survival had not been reached. At a median follow-up of 9.4 months, 86% of the patients with a response continued treatment or had undergone curative surgery. At 1 year, 71% of the responses were ongoing and 55% of the patients remained progression-free. The most common adverse events of any grade were fatigue, vomiting and abnormal liver function studies. None of the patients on Larotrectinib discontinued therapy due to a drug-related adverse event.
It was concluded that TRK fusions defined a unique molecular subgroup of advanced solid tumors in children and adults and Larotrectinib had marked and durable antitumor activity in patients with TRK fusion-positive cancer, regardless of the age of the patient or tumor type. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. Drilon A, Laetsch TW, Kummar S, et al. N Engl J Med 2018; 378:731-739
FoundationOne CDx (F1CDx)
The FDA on November 30, 2017, granted marketing approval to the FoundationOne CDx, a Next Generation Sequencing (NGS) based in vitro diagnostic (IVD) to detect genetic mutations in 324 genes and two genomic signatures in any solid tumor type. The test can also identify which patients with Non-Small Cell Lung Cancer (NSCLC), melanoma, breast cancer, colorectal cancer, or ovarian cancer may benefit from 15 different FDA-approved targeted treatment options. This test is offered by Foundation Medicine, Inc.
Platelet Transfusion for Patients with Cancer American Society of Clinical Oncology Clinical Practice Guideline Update
SUMMARY: The ASCO convened an Expert Panel and updated evidence-based guidance on the use of platelet transfusion in patients with cancer. This guideline updates is based on a systematic review of the medical literature published from September 1, 2014, through October 26, 2016 and this review builds on two 2015 systematic reviews that were conducted by the AABB and the International Collaboration for Transfusion Medicine Guidelines. This ASCO guideline replaces the previous ASCO platelet transfusion guideline published initially in 2001. The updated ASCO review included 24 more recent publications which included 3 clinical practice guidelines, 8 systematic reviews, and 13 observational studies.
Target Population: Adults and children (4 months of age or older) with hematologic malignancies, solid tumors, or hypoproliferative thrombocytopenia.
Target Audience: Clinician’s administering intensive therapies to patients with cancer.
Clinical Questions and Recommendations:
(1) How should platelets for transfusion be prepared?
Platelets can be prepared either by separation of units of platelet concentrates from whole blood using either the buffy coat or the platelet-rich plasma method, which can then be pooled before administration, or by apheresis from single donors. Studies have shown that the post-transfusion increments, hemostatic benefit, and adverse effects are similar with any of these platelet products and they can be used interchangeably. However, single-donor platelets from selected donors are necessary when histocompatible platelet transfusions are needed.
(2) In what circumstances should providers take steps to prevent Rh alloimmunization resulting from platelet transfusion?
Prevention of RhD alloimmunization resulting from platelet transfusions to RhD-negative recipients can be achieved either through the exclusive use of platelet products collected from RhD-negative donors or via anti-D immunoprophylaxis. These approaches may be used for female children and female adults of child-bearing potential being treated with curative intent. However, because of the low rate of RhD alloimmunization in patients with cancer, these approaches need not be applied universally.
(3) In what circumstances should providers use leukoreduced blood products to prevent alloimmunization?
Providing leukoreduced blood products to patients with Acute Myeloid Leukemia from the time of diagnosis is appropriate, as the incidence of alloantibody-mediated refractoriness to platelet transfusion can be decreased in patients receiving induction chemotherapy, when both platelet and RBC products are leukoreduced before transfusion. It is likely that alloimmunization can also be decreased in patients with other types of leukemia and in other patients with cancer who are receiving chemotherapy. The same holds true for patients with Aplastic Anemia, and Myelodysplasia not receiving chemotherapy, in the same time periods that the transfusions are being administered. In the United States and in several other countries, majority of blood products are leukoreduced at the time of blood collection and component preparation. Prestorage leukoreduction can result in a substantial reduction in transfusion reactions and in transmission of cytomegalovirus (CMV) infection
(4) Should platelet transfusions be given prophylactically or therapeutically?
Prophylactic platelet transfusion should be administered to patients with thrombocytopenia resulting from impaired bone marrow function to reduce the risk of hemorrhage, when the platelet count falls below a predefined threshold level. This threshold level for transfusion varies according to the patient’s diagnosis, clinical condition, and treatment modality.
(5) What is the appropriate threshold for prophylactic platelet transfusion in patients with hematologic malignancies?
The Panel recommends a threshold of less than 10×109/L for prophylactic platelet transfusion in patients receiving therapy for hematologic malignancies. Transfusion at higher levels may be advisable in patients with signs of hemorrhage, high fever, hyperleukocytosis, rapid fall of platelet count, or coagulation abnormalities (eg, acute promyelocytic leukemia) and in those undergoing invasive procedures or in circumstances in which platelet transfusions may not be readily available in case of emergencies, as might be the case for outpatients who live at a distance from the treatment center.
(6) What is the appropriate threshold for prophylactic platelet transfusion in the setting of Hematopoietic Stem Cell Transplantation (HSCT)?
The Panel recommends a threshold of less than 10×109/L for prophylactic platelet transfusion in adult and pediatric patients undergoing allogeneic HSCT. Prophylactic platelet transfusion may be administered at higher counts based on clinician judgment. In adult recipients of autologous HSCT, randomized trials have demonstrated similar rates of bleeding with decreased platelet usage when patients are transfused at the first sign of bleeding rather than prophylactically, and this approach may be used in experienced centers. This recommendation is not generalizable to pediatric patients.
(7) Is there a role for prophylactic platelet transfusion in patients with chronic, stable, severe thrombocytopenia who are not receiving active treatment?
Patients with chronic, stable, severe thrombocytopenia, such as individuals with Myelodysplasia or Aplastic Anemia, who are not receiving active treatment may be observed without prophylactic transfusion, reserving platelet transfusions for episodes of hemorrhage or during times of active treatment.
(8) What is the appropriate threshold for prophylactic platelet transfusion in patients with solid tumors?
The risk of bleeding in patients with solid tumors during chemotherapy-induced thrombocytopenia is related to the depth and duration of the platelet nadir, although other factors contribute as well. The Panel recommends a threshold of less than 10×109/L for prophylactic platelet transfusion. Platelet transfusion at higher levels is appropriate in patients with active localized bleeding, which can sometimes be seen in patients with necrotic tumors.
(9) At what platelet count can surgical or invasive procedures be performed?
The Panel recommends a threshold of 40×109/L to 50×109/L for performing major invasive procedures in the absence of associated coagulation abnormalities. Certain procedures, such as bone marrow aspirations and biopsies, and insertion or removal of central venous catheters, can be performed safely at counts 20×109/L or more. If platelet transfusions are administered before a procedure, it is critical that a post-transfusion platelet count be obtained to prove that the desired platelet count level has been reached. Platelet transfusions should also be available on short notice, in case intraoperative or postoperative bleeding occurs. For alloimmunized patients, histocompatible platelets must be available in these circumstances.
(10) When and how should patients be monitored for refractoriness to platelet transfusion?
The Panel recommends that when refractoriness is suspected, platelet counts should be performed 10-60 minutes after transfusion. Because patients may have a poor increment to a single transfusion and yet have excellent platelet increments with subsequent transfusions, a diagnosis of refractoriness to platelet transfusion should only be made when at least two transfusions of ABO-compatible units, stored for less than 72 hours, result in poor increments (less than 5000/microliter).
(11) How should refractoriness to platelet transfusion be managed?
Alloimmunization is usually due to antibody against HLA antigens and only rarely to platelet-specific antigens. Patients with alloimmune-refractory thrombocytopenia, as defined previously, are best managed with platelet transfusions from histocompatible donors matched for HLA-A and HLA-B antigens. For patients( 1) whose HLA type cannot be determined, (2) who have uncommon HLA types for whom suitable donors cannot be identified, or (3) who do not respond to HLA-matched platelets, histocompatible platelet donors can often be identified using platelet cross-matching techniques. In many patients, these two techniques are complementary.
Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Schiffer CA, Bohlke K, Delaney M, et al. J Clin Oncol. 2017 Nov 28:JCO2017761734. doi: 10.1200/JCO.2017.76.1734. [Epub ahead of print]
FDA Approves FoundationOne CDx Next Generation Sequencing Based Assay to Tailor Cancer Therapies
SUMMARY: The FDA on November 30, 2017, granted marketing approval to FoundationOne CDx (F1CDx), a Next Generation Sequencing (NGS) based, in vitro diagnostic (IVD) assay, to detect genetic mutations in 324 genes and two genomic signatures, in any solid tumor type. The test can also identify which patients with Non Small Cell Lung Cancer (NSCLC), Melanoma, Breast cancer, ColoRectal cancer, or Ovarian cancer may benefit from 15 different FDA-approved targeted treatment options.
The basic premise of cancer genomics is that cancer is caused by somatically acquired mutations, and is therefore a disease of the genome. Tumor genomic profiling enables the identification of specific genomic alterations and thereby can provide personalized treatment options with targeted therapies that are specific for those molecular targets. A genomic test can be performed on a tumor specimen or on cell-free DNA in plasma (“liquid biopsy”) or an ImmunoHistoChemistry (IHC) test can be performed on tumor tissue for protein expression that demonstrates a genomic variant known to be a drug target, or to predict sensitivity to a chemotherapeutic drug.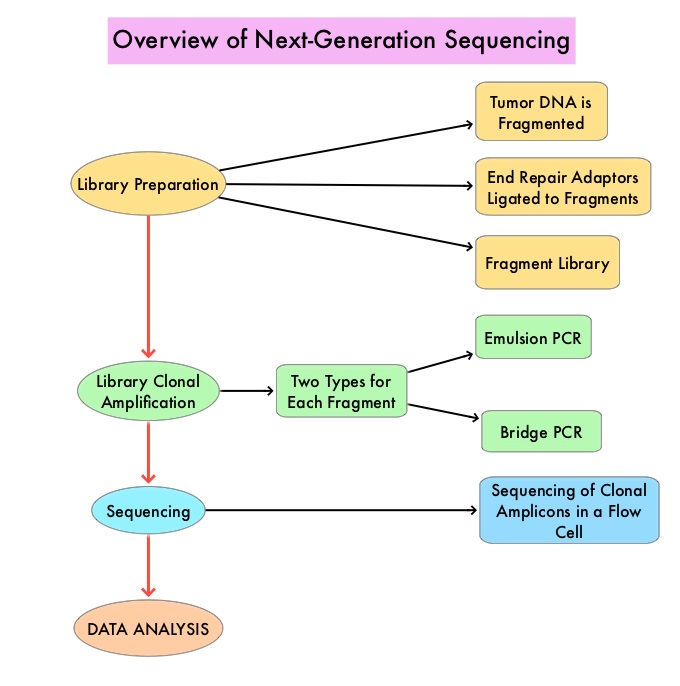
Next-Generation Sequencing (NGS) platforms or second-generation sequencing, unlike the first-generation sequencing, known as Sanger sequencing, perform massively parallel sequencing, which allows sequencing of millions of fragments of DNA from a single sample. With this high-throughput sequencing, the entire genome can be sequenced in less than 24 hours. This is in contrast to Sanger sequencing technology which has required over a decade to decipher the human genome. There are a number of different NGS platforms using different sequencing technologies and NGS can be used to sequence and systematically study the cancer genomes in their entirety or specific areas of interest in the genome or small numbers of individual genes. Recently reported genomic profiling studies, performed in patients with advanced cancer suggest that actionable mutations are found in 20-40% of patients’ tumors.
The application for F1CDx , was reviewed by the FDA using a coordinated, cross-agency approach and clinical performance of the test was established by comparing F1CDx to previously FDA-approved companion diagnostic tests, that are currently used to determine patient eligibility for certain treatments. It was noted that F1CDx assay’s ability to detect select mutation types (substitutions and short insertions and deletions) representative of the entire 324 gene panel was accurate approximately 94.6% of the time. This 324 gene panel included EGFR, KRAS, BRAF, BRCA1/2, ALK, and several other genes with emerging therapies, such as NTRK1/2/3. This assay can additionally detect MicroSatellite Instability (MSI) and Tumor Mutational Burden, which can predict response to immunotherapy.
The FDA noted that this is the first device with the FDA’s “Breakthrough Device” designation to complete the PreMarket Approval (PMA) process, and it is the second IVD authorized under the FDA and Centers for Medicare & Medicaid Services’ (CMS) Parallel Review program. Under this program, the CMS issued a proposed national coverage determination of the F1CDx for Medicare beneficiaries with recurrent, metastatic, or advanced Stage IV cancer, who have not been previously tested using NGS technology, and who continue to remain candidates for further therapy. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm587387.htm
Alcohol and Cancer A Statement of the American Society of Clinical Oncology
Alcohol consumption is an established risk factor for several malignancies, and is a potentially modifiable risk factor for cancer. The International Agency for Research on Cancer (IARC), a branch of WHO, classified alcohol as a group 1 carcinogen. The American Heart Association, American Cancer Society, and US Department of Health and Human Services all recommend that men limit intake to one to two drinks per day and women to one drink per day. People who do not currently drink alcohol should not start for any reason. There is a clear association between alcohol and upper aerodigestive tract cancers (larynx, esophagus, and oral cavity/pharynx). A recent meta-analysis of cohort studies among 209,597 cancer survivors showed an 8% increase in overall mortality and a 17% increased risk for recurrence in the highest versus lowest alcohol consumers. The benefit of alcohol consumption on cardiovascular health likely has been overstated and the net effect of alcohol is harmful. Alcohol consumption should therefore not be recommended to prevent cardiovascular disease or all-cause mortality.
