Late Breaking Abstract – ASCO 2016: Liquid Biopsy Can Rapidly Detect Certain Gene Mutations with High Specificity
SUMMARY: The FDA approved the first “Liquid Biopsy” test on June 1, 2016 for the detection of exon 19 deletions or exon 21 (L858R) substitution mutations in the Epidermal Growth Factor Receptor (EGFR) gene. On the heels of this approval, Zill and colleagues reported the results of the largest liquid biopsy study ever conducted thus far. It has been well established that treatment with EGFR TKIs results in superior outcomes, for patients with tumors harboring exon 19 deletions and exon 21 mutations. The application of precision medicine with targeted therapy requires detection of molecular abnormalities in a tumor specimen, following progression or recurrence. Archived biopsy specimens may not be helpful, as it is important to identify additional mutations in the tumor at the time of recurrence or progression, in order to plan appropriate therapy. Further, recurrent tumors may be inaccessible for a safe biopsy procedure or the clinical condition of the patient may not permit a repeat biopsy. Additionally, the biopsy itself may be subject to sampling error due to tumor heterogeneity. Genotyping cell free DNA in the plasma, also called liquid biopsy, can potentially overcome the shortcomings of repeat biopsies and tissue genotyping, allowing the detection of many more targetable gene mutations, thus resulting in better evaluation of the tumor genome landscape.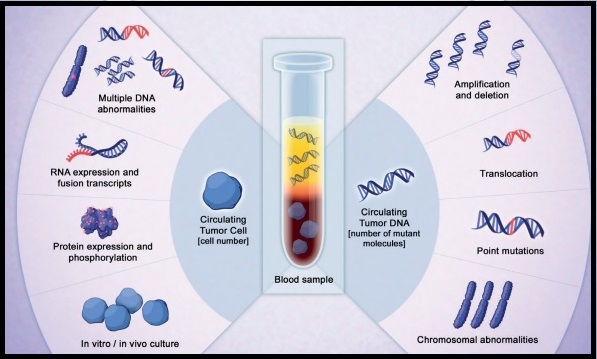
The authors in this study utilized Next Generation Sequencing (NGS) of circulating tumor DNA (ctDNA), isolated from plasma specimens (liquid biopsy specimens) of 15,191 patients of whom 37% had advanced lung cancer, 14% had breast cancer, 10% had colorectal cancer and 39% had other malignancies. Seventy genes were targeted and accuracy of ctDNA sequencing was assessed by comparing with matched tissue tests for 386 patients and frequencies of somatic ctDNA alterations per gene were compared to those previously described in tissue sequencing projects such as data from The Cancer Genome Atlas (TCGA).
It was noted that the ctDNA mutation patterns were highly concordant with tissue analysis as reported by the TCGA. The overall accuracy of ctDNA sequencing in comparison with matched tissue tests was 87% and the accuracy increased to 98% when blood and tumor were collected less than six months apart. Pearson Correlation between sets of data is a measure of how well these sets are related. Between 0.5 and 1.0 is considered high correlation. Pearson correlation for TP53 gene was 0.94, for KRAS was 0.99 and for PIK3CA was 0.99.
The researchers commented on the clinical outcome benefits using liquid biopsy, in four distinct groups:
1) Testing for actionable mutations (ALK fusion, EGFR or BRAF activating mutations in lung; ERBB2 amplification in gastric cancer) in cases with insufficient tissue quantity.
2) Testing for actionable resistance mutations (MET amplification or EGFR T790M in lung cancer), at the time of progression.
3) Genomic evolution upon progression such as ERBB2-amplified metastatic breast cancer in patients with triple negative primary tumor.
4) Tumors with genotypes that need more extensive driver mutation testing such as BRAF V600E in lung.
The authors concluded that there is a high correlation between ctDNA plasma samples and tissue testing with the exception of resistance mutations such as EGFR T790M mutation which evolve while on anti-EGFR inhibitor therapy and consequently may not correlate with the TCGA, probably because patients in the tissue-based population had not yet received the anti-EGFR inhibitor therapy that promotes the mutation. Patients who received treatment based on ctDNA findings also experienced better clinical outcomes. Zill OA, Mortimer S, Banks KC, et al Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl; abstr LBA11501).

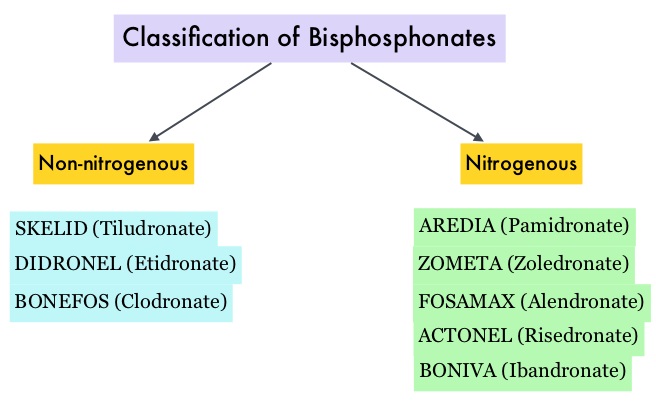 Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.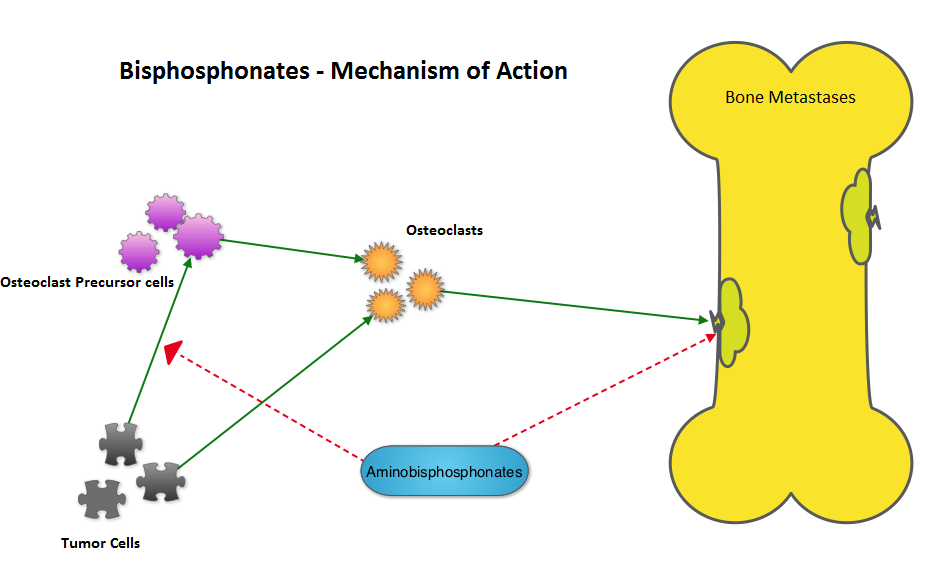 The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.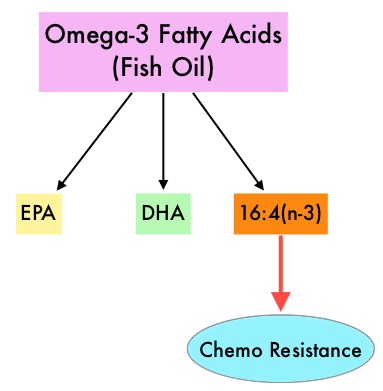 Fish oil has relevant levels of fatty acid 16:4(n-3) and preclinical models have shown that the fish oil neutralized the antitumor activity of chemotherapy, thus conferring drug resistance. With this preclinical information and given that cancer patients frequently use fish oil supplements, the authors evaluated the effect of fish oil intake in healthy volunteers, on the plasma levels of fatty acid 16:4(n-3), which has been shown to induce resistance to chemotherapeutic agents. The researchers first conducted a survey to determine what percentage of cancer patients undergoing treatment at a University Medical Center in the Netherlands were taking fish oil supplements. They also analyzed fatty acid 16:4(n-3) content, in 3 brands of fish oil supplements and 4 often consumed species of fish. The authors then randomly selected 30 healthy volunteers for the fish oil study and 20 healthy volunteers for the fish consumption study and the plasma levels of fatty acid 16:4(n-3) was measured after they consumed fish oil or fish, for a period of 2 weeks. They noted that 11% of the cancer patients in their study reported using omega-3 supplements. All fish oils tested contained amounts of fatty acid 16:4(n-3) ranging from 0.2 to 5.7 μM and this was adequate to induce chemoresistance to a variety of chemotherapeutic agents. They noted that there was a significant rise in the plasma 16:4(n-3) fatty acid levels in the healthy volunteers after they consumed fish oil supplements and fish, with high levels of fatty acid 16:4(n-3). Herring and Mackerel fish contained high levels of fatty acid 16:4(n-3), in contrast to Salmon and Tuna. The authors concluded that based on this preclinical data it is best to avoid fish oils and fish such as Herring and Mackerel in the 48 hours surrounding chemotherapy, as the high plasma 16:4(n-3) fatty acid levels may negate the effects of chemotherapy. These recommendations have been adopted by the Dutch Cancer Society and by the Dutch National Working Group for Oncologic Dieticians. Increased Plasma Levels of Chemoresistance-Inducing Fatty Acid 16:4(n-3) After Consumption of Fish and Fish Oil. Daenen LGM, Cirkel GA, Houthuijzen JM, et al. JAMA Oncol. 2015;1:350-358
Fish oil has relevant levels of fatty acid 16:4(n-3) and preclinical models have shown that the fish oil neutralized the antitumor activity of chemotherapy, thus conferring drug resistance. With this preclinical information and given that cancer patients frequently use fish oil supplements, the authors evaluated the effect of fish oil intake in healthy volunteers, on the plasma levels of fatty acid 16:4(n-3), which has been shown to induce resistance to chemotherapeutic agents. The researchers first conducted a survey to determine what percentage of cancer patients undergoing treatment at a University Medical Center in the Netherlands were taking fish oil supplements. They also analyzed fatty acid 16:4(n-3) content, in 3 brands of fish oil supplements and 4 often consumed species of fish. The authors then randomly selected 30 healthy volunteers for the fish oil study and 20 healthy volunteers for the fish consumption study and the plasma levels of fatty acid 16:4(n-3) was measured after they consumed fish oil or fish, for a period of 2 weeks. They noted that 11% of the cancer patients in their study reported using omega-3 supplements. All fish oils tested contained amounts of fatty acid 16:4(n-3) ranging from 0.2 to 5.7 μM and this was adequate to induce chemoresistance to a variety of chemotherapeutic agents. They noted that there was a significant rise in the plasma 16:4(n-3) fatty acid levels in the healthy volunteers after they consumed fish oil supplements and fish, with high levels of fatty acid 16:4(n-3). Herring and Mackerel fish contained high levels of fatty acid 16:4(n-3), in contrast to Salmon and Tuna. The authors concluded that based on this preclinical data it is best to avoid fish oils and fish such as Herring and Mackerel in the 48 hours surrounding chemotherapy, as the high plasma 16:4(n-3) fatty acid levels may negate the effects of chemotherapy. These recommendations have been adopted by the Dutch Cancer Society and by the Dutch National Working Group for Oncologic Dieticians. Increased Plasma Levels of Chemoresistance-Inducing Fatty Acid 16:4(n-3) After Consumption of Fish and Fish Oil. Daenen LGM, Cirkel GA, Houthuijzen JM, et al. JAMA Oncol. 2015;1:350-358