SUMMARY: The U.S. Food and Drug Administration on September 2, 2015, approved VARUBI® (Rolapitant) to prevent delayed phase Chemotherapy Induced Nausea and Vomiting (CINV). Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization. Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment, whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on Serotonin (5-hydroxytryptamine-5HT3) and its receptors, with the chemotherapeutic agents stimulating the release of Serotonin from the enterochromaffin cells of the small intestine. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are also located centrally in the Chemoreceptor Trigger Zone of the area Postrema. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. VARUBI® is a substance P/Neurokinin-1 (NK-1) receptor antagonist.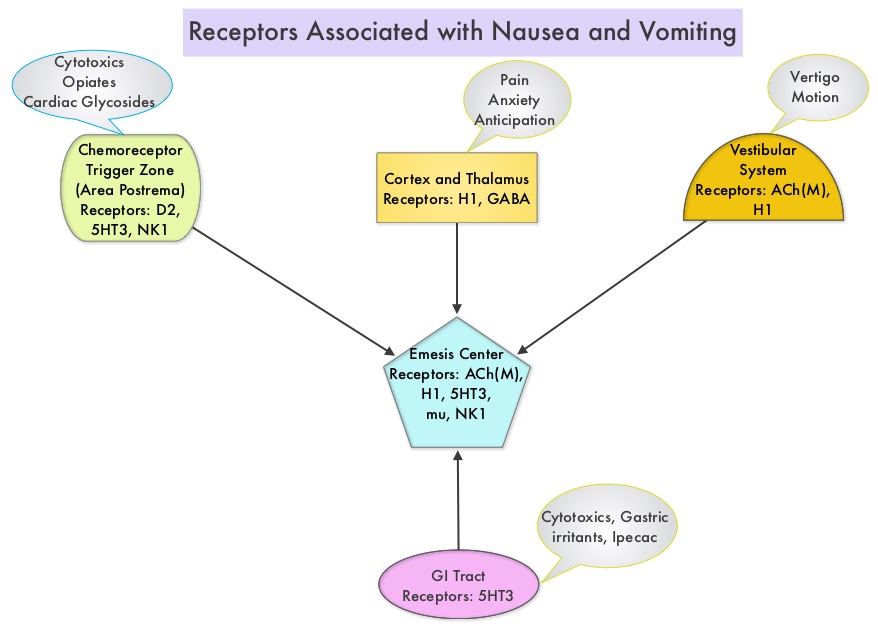
The safety and efficacy of VARUBI® were established in three randomized, double-blind, controlled clinical trials where VARUBI® in combination with KYTRIL® (Granisetron) and Dexamethasone was compared with placebo, KYTRIL® and Dexamethasone (control therapy), in more than 2500 patients receiving a moderately or highly emetic chemotherapy regimen. HEC Study 1 and HEC Study 2 included Cisplatin Based Highly Emetogenic Chemotherapy (HEC). Chemotherapy regimens included more than 60 mg/m2 of Cisplatin. In HEC Study 1, 532 patients were randomized to receive either antiemetic regimen with VARUBI® (N =266) or control therapy (N =266). In HEC Study 2, a total of 555 patients were randomized to receive either antiemetic regimen with VARUBI® (N =278) or control therapy (N =277). MEC Study 3 included Moderately Emetogenic Chemotherapy and combinations of Anthracycline and Cyclophosphamide chemotherapy. A total of 1369 patients were randomized in this study to receive either antiemetic regimen with VARUBI® (N =684) or control therapy (N =685). Patients in these trials received either VARUBI® 180 mg PO or placebo at 1 to 2 hours before administration of Highly Emetogenic Chemotherapy. All patients received intravenous KYTRIL® 10 μg/kg IV and Dexamethasone 20 mg PO on day 1 and Dexamethasone 8 mg PO twice daily on days 2 to 4 for up to six cycles, with each cycle lasting a minimum of 14 days. The primary endpoint in all three studies was complete response (defined as no emetic episodes and no rescue medication) in the delayed phase (25 to 120 hours) post chemotherapy.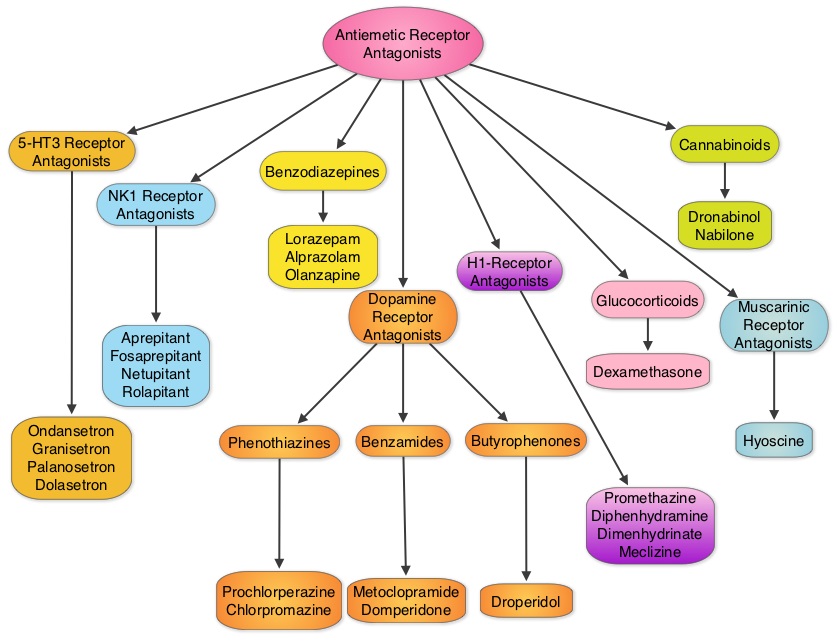
It was noted that a significantly greater proportion of patients receiving antiemetic regimen with VARUBI® had complete responses in the delayed phase than did patients in the control therapy group – HEC Study 1: 72.7% vs 58.4% (P<0.001), HEC Study 2: 70.1% vs 61.9% (P=0.043) and MEC Study 3: 71.3% vs 61.6% (P<0.001). The most common adverse events in patients treated with VARUBI® included neutropenia, hiccups, decreased appetite and dizziness. It was concluded from these three trials that VARUBI® when combined with a 5-HT3 receptor antagonist such as KYTRIL® and a corticosteroid, significantly prevented delayed Chemotherapy Induced Nausea and Vomiting.
1) Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Rapoport BL, Chasen MR, Gridelli C, et al. The Lancet Oncology 2015;16:1079-1089
2) Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving moderately emetogenic chemotherapy (MEC). Schnadig ID, Modiano MR, Poma A, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9633)

