SUMMARY: Brain metastases from an extracranial primary, occur in approximately 15% of cancer patients and this is estimated to be about 400,000 to 600,000 patients annually. The incidence of brain metastases has been on the rise with the availability of more effective systemic therapies and better control of systemic disease. The most frequent malignancies associated with brain metastases include Lung cancer, Breast cancer and Melanoma. Majority of the patients with brain metastases have synchronous extracerebral metastases. A significant number of patients present with solitary or fewer than 3 brain metastases and they may be amenable to focal therapeutic interventions. However, Whole Brain Radiation Therapy (WBRT) has been the standard treatment strategy since the 1950’s. It is also well recognized that WBRT can be associated with neurocognitive dysfunction. Stereotactic RadioSurgery (SRS) is a non-surgical procedure that allows delivery of significantly higher doses of precisely focused radiation to the tumor, compared to conventional radiation therapy, with less collateral damage to the surrounding normal tissue. The technologies used for SRS include GAMMA KNIFE® which uses highly focused gamma rays, Proton Beam therapy which uses ionized hydrogen or Protons, Linear Accelerator and CYBER KNIFE® which use Photons, to target the tumor tissue.
NCCTG N0574 is a federally funded, randomized phase III clinical trial, designed to determine whether cognitive deterioration occurred less frequently with SRS alone compared to SRS followed by WBRT, in patients with 1-3 brain metastases. In this study, 213 patients with 1-3 brain metastases, each measuring less than 3 cm by contrast MRI, were enrolled and randomized to SRS alone or SRS plus WBRT. All patients underwent cognitive testing before and after treatment. Sixty eight percent (68%) of the enrolled patients had a Lung primary and the median age was 60 years. Baseline characteristics were similar in both treatment groups. The median follow up was 7.2 months. The authors used several tools to assess cognitive dysfunction and the primary endpoint was the cognitive decline at 3 months following treatment. It was noted that at 3 months, with the addition of WBRT to SRS, 91.7% of patients experienced cognitive decline compared with 63.5% for those receiving SRS alone (P=0.0007) and there was statistically significant decline in immediate recall, delayed recall and verbal fluency, in the SRS plus WBRT group. Patients who received SRS plus WBRT also reported significantly worse Quality of Life. There was however better intracranial tumor control at 6 and 12 months with SRS plus WBRT compared to SRS alone (P< 0.001), but this local control had no significant impact on the median Overall Survival (OS), with similar OS outcomes noted in both treatment groups (P=0.93). The authors concluded that the addition of WBRT to SRS can result in significant decline in neurocognitive function, without any Overall Survival benefit, compared to SRS alone. It is therefore recommended that patients with newly diagnosed brain metastases amenable to SRS, be closely monitored after SRS, with consideration given to WBRT, at the time of symptomatic progression. NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. Brown PD, Asher AL, Ballman KV, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA4)

 Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s
Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s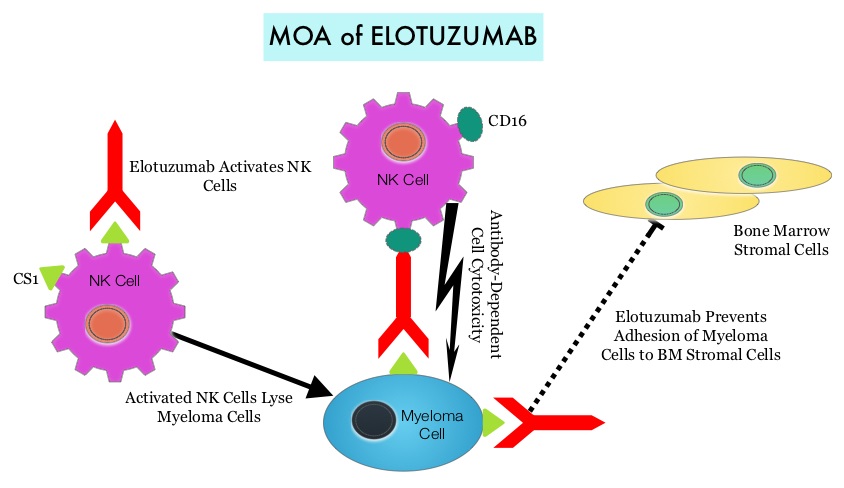 By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s
By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s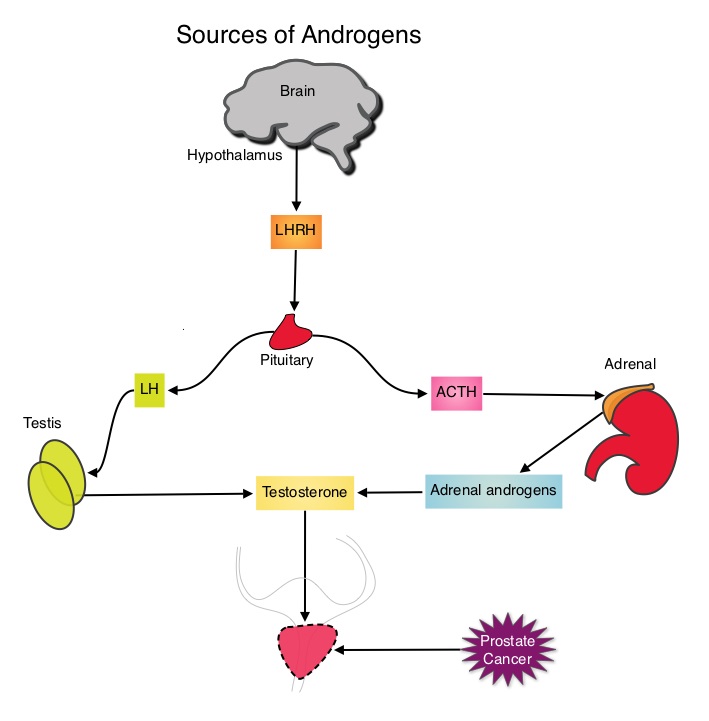 The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2 This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s
This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s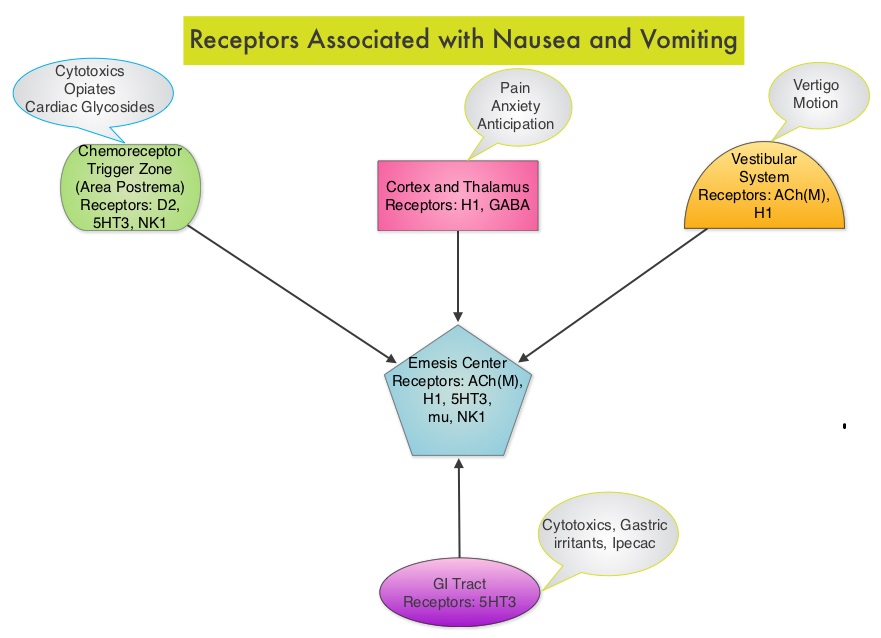 Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.
Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.  Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s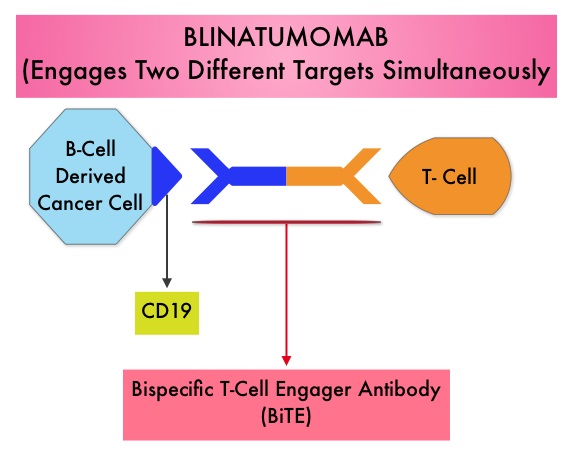 The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s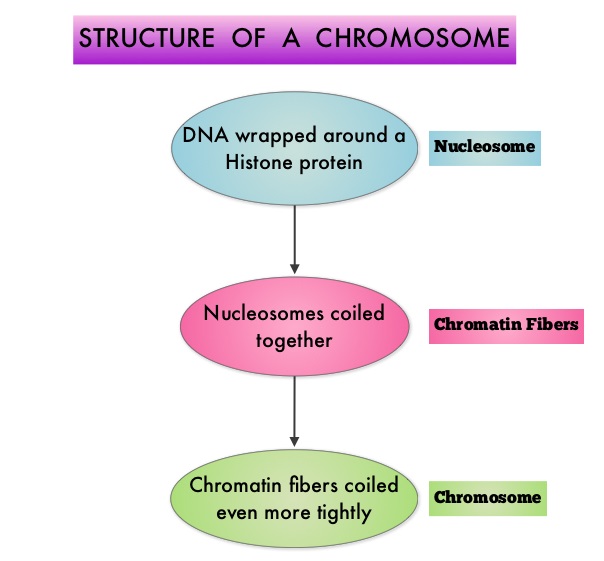 To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.
To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.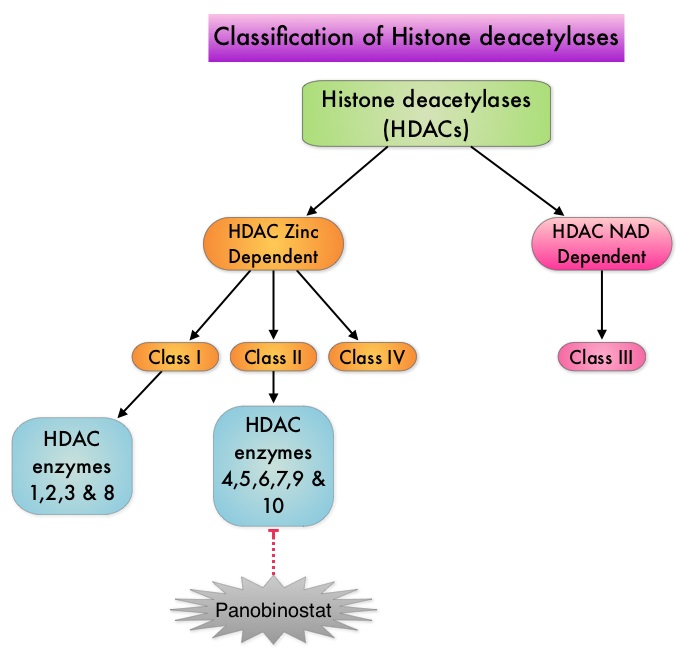 Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s
Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s