SUMMARY:Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 24,050 new cases will be diagnosed in 2014 and 11,090 will die of the disease. The authors in the PANORAMA I trial evaluated the outcomes in previously treated advanced multiple myeloma patients, by taking advantage of the synergy between Bortezomib (VELCADE®), a proteosome inhibitor and Panobinostat, a histone deacetylase (HDAC) inhibitor and treating these patients with a combination of these two agents. HDACs are a family of enzymes that play an important role in the regulation of gene expression.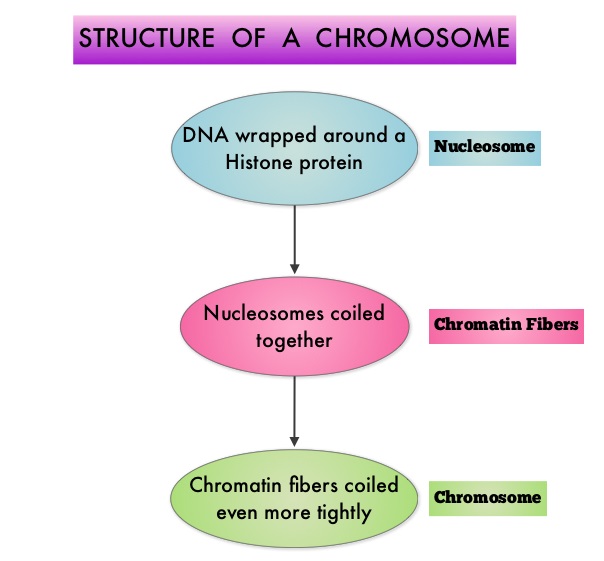 To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.
To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.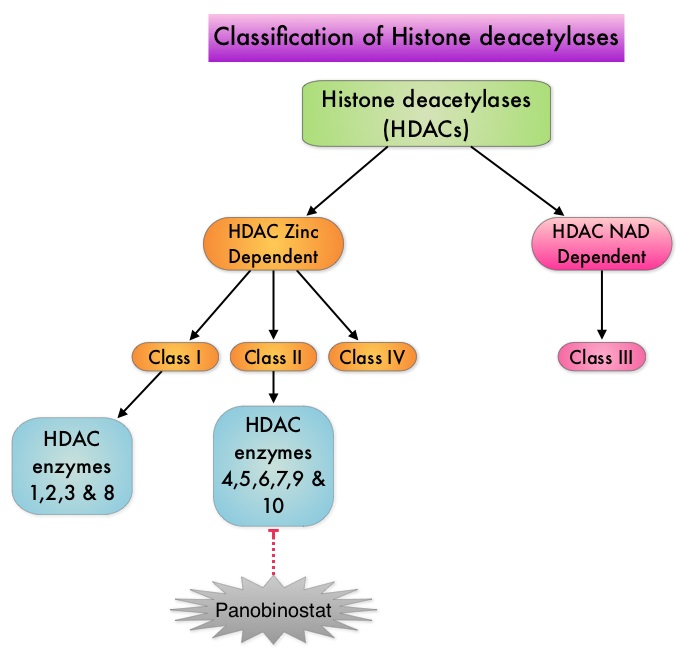 Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s
Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s

