SUMMARY:The FDA in July 2014, granted Breakthrough Therapy Designation to Blinatumomab, a bispecific T cell engager (BiTE) antibody, for adults with Philadelphia-negative (Ph-) Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia (ALL). BiTE® technology engages the body’s immune system to detect and target malignant cells. These modified antibodies are designed to engage two different targets simultaneously, thereby placing the T cells within reach of the targeted cancer cell and facilitating apoptosis of the cancer cell. BiTE antibodies are currently being investigated to treat a wide variety of malignancies. Blinatumomab is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas.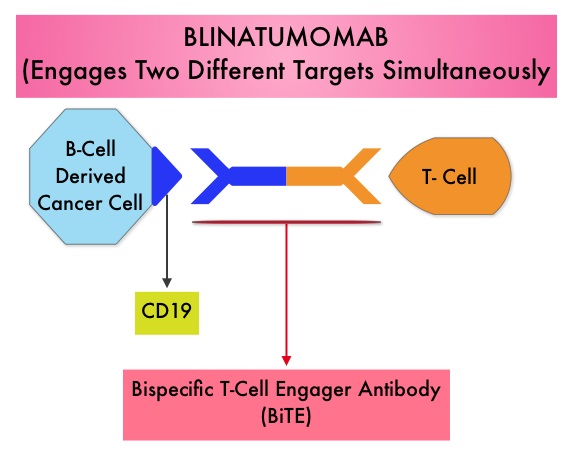 The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s

