SUMMARY: Acute Myeloid Leukemia (AML) is generally a disease of the elderly and the average age of a patient at the time of diagnosis is about 66 years. According to the American Cancer Society, approximately 18,860 new cases of AML will be diagnosed in 2014 and 10,460 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy. 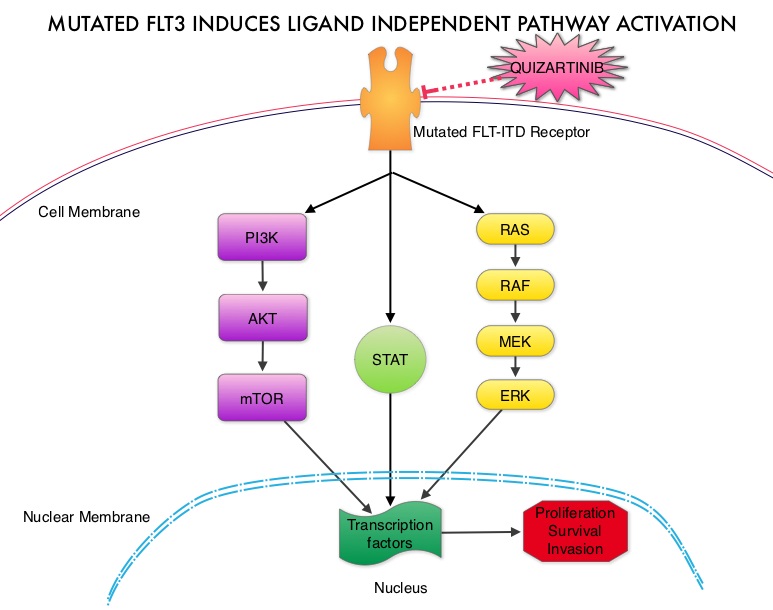 The fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by a tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. Several therapeutic agents are being developed to target FLT3 mutations. Quizartinib (AC220) is an oral tyrosine kinase inhibitor, which has demonstrated activity in patients with both wild type FLT3 as well as those with FLT3-ITD mutations. In this randomized, open label phase II study, the authors evaluated the efficacy and safety of two different, lower dosages of Quizartinib, in patients 18 years of age or older, with FLT3-ITD positive, relapsed or refractory AML. Seventy six patients (N=76) were randomized to receive either Quizartinib 30 mg/day (Group A) or Quizartinib 60 mg/day (Group B), given orally and continuously, during a 28 day treatment cycle. Treatment was continued until relapse, intolerance or Hematopoietic Stem Cell Transplantation (HSCT). Both groups were well balanced except for age over 60 years (42% Group A, 26% Group B) and the percentage with secondary AML (8% Group A, 18% Group B). The composite Complete Remission (CRc) rate included Complete Remission (CR), Complete Remission with incomplete platelet recovery (CRp), and Complete Remission with incomplete hematologic recovery (CRi). The CRc rate in both groups A and B was 47% and the Overall Response Rate (CRc + Partial Response (PR)) was 61% in Group A and 71% in Group B. Further, 32% of patiens in Group A and 42% in Group B were able to undergo HSCT, after achieving CRc or PR. The most common treatment related adverse events were diarrhea (18%), febrile neutropenia (16%), and QT prolongation (15%). The QT prolongation rate was significantly less with lower doses of Quizartinib, as was used in this study, compared to what was noted with higher doses of Quizartinib utilized in other trials. The authors concluded that Quizartinib is highly effective in relapsed and refractory AML patients with FLT3-ITD mutations, with an acceptable safety profile. Schiller GJ, Tallman MS, Goldberg SL, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7100)
The fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by a tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. Several therapeutic agents are being developed to target FLT3 mutations. Quizartinib (AC220) is an oral tyrosine kinase inhibitor, which has demonstrated activity in patients with both wild type FLT3 as well as those with FLT3-ITD mutations. In this randomized, open label phase II study, the authors evaluated the efficacy and safety of two different, lower dosages of Quizartinib, in patients 18 years of age or older, with FLT3-ITD positive, relapsed or refractory AML. Seventy six patients (N=76) were randomized to receive either Quizartinib 30 mg/day (Group A) or Quizartinib 60 mg/day (Group B), given orally and continuously, during a 28 day treatment cycle. Treatment was continued until relapse, intolerance or Hematopoietic Stem Cell Transplantation (HSCT). Both groups were well balanced except for age over 60 years (42% Group A, 26% Group B) and the percentage with secondary AML (8% Group A, 18% Group B). The composite Complete Remission (CRc) rate included Complete Remission (CR), Complete Remission with incomplete platelet recovery (CRp), and Complete Remission with incomplete hematologic recovery (CRi). The CRc rate in both groups A and B was 47% and the Overall Response Rate (CRc + Partial Response (PR)) was 61% in Group A and 71% in Group B. Further, 32% of patiens in Group A and 42% in Group B were able to undergo HSCT, after achieving CRc or PR. The most common treatment related adverse events were diarrhea (18%), febrile neutropenia (16%), and QT prolongation (15%). The QT prolongation rate was significantly less with lower doses of Quizartinib, as was used in this study, compared to what was noted with higher doses of Quizartinib utilized in other trials. The authors concluded that Quizartinib is highly effective in relapsed and refractory AML patients with FLT3-ITD mutations, with an acceptable safety profile. Schiller GJ, Tallman MS, Goldberg SL, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7100)

