SUMMARY: The FDA in November 2018 approved XOSPATA® (Gilteritinib) for treatment of adult patients who have relapsed or refractory Acute Myeloid Leukemia (AML) with a FLT3 mutation as detected by an FDA-approved test. The FDA also approved an expanded indication for a companion diagnostic, to include use with XOSPATA®. The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe Technologies, Inc., is used to detect the FLT3 mutation in patients with AML. The American Cancer Society estimates that in 2019, 21,450 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,920 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. A significant percentage of patients with newly diagnosed AML are not candidates for intensive chemotherapy. Even with the best available therapies, the 5 year Overall Survival in patients 65 years of age or older is less than 5%.
Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy alone. More importantly, with the understanding of molecular pathology of AML, personalized and targeted therapies are becoming an important part of the AML treatment armamentarium.
The Fms-Like Tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. FLT3 mutations occur most often as Internal Tandem Duplications (FLT3-ITD) or point mutations at codon D835 (FLT3-Tyrosine Kinase Domain or TKD). Approximately 80% of AML patients with a FLT3 mutation will have the FLT3-ITD, and about 15% will have both FLT3-ITD and FLT3-TKD, and about 5% will have FLT3-TKD alone. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations are predicted to have poor outcomes with shorter remission duration and significantly decreased leukemia free and Overall Survival. FLT3-TKD can confer resistance to other Tyrosine Kinase Inhibitors.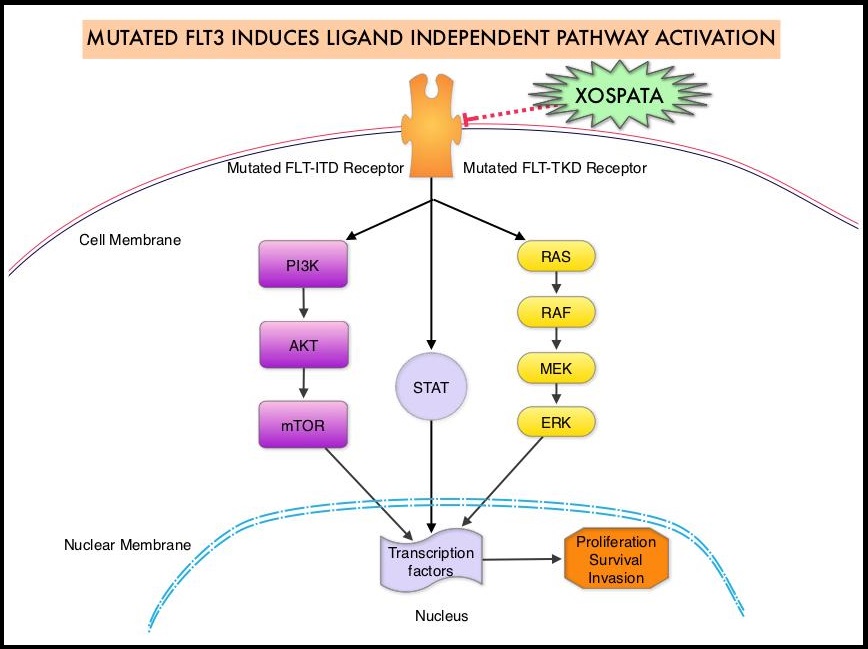
XOSPATA® (Gilteritinib) is a highly selective FLT3/AXL Tyrosine Kinase Inhibitor with activity against both FLT3-ITD and FLT3-TKD mutations. This unlike RYDAPT® (Midostaurin), which is a not selective and is a multikinase inhibitor and inhibits FLT3, PDGFR, c-KIT (CD 117), VEGFR, and protein kinase C. The approval of XOSPATA® was based on an interim analysis of the ADMIRAL trial, which included 138 adult patients with relapsed or refractory AML having a FLT3 ITD, D835, or I836 mutation as detected by the LeukoStrat CDx FLT3 Mutation Assay. XOSPATA® was given orally at a dose of 120 mg daily until unacceptable toxicity or lack of clinical benefit. The median patient age was 60 years and 59% of patients had untreated relapsed AML, 41% had primary refractory AML and 20% of patients had prior Stem Cell transplantation. With regards to FLT3 mutation status, 121 patients had ITD alone, 12 patients had TKD alone, and 5 patients had ITD and TKD.
At the time of interim analysis, the complete remission (CR)/Complete Remission with partial hematologic recovery (CRh) rate was 21%. The median duration of CR/CRh was 4.6 months. The rate of conversion from transfusion dependence to transfusion independence was 31.1% for any 56 day post-baseline period. For those patients who were independent of both RBC and platelet transfusions at baseline, 53.1% remained transfusion-independent during any 56-day post-baseline period. . For patients who achieved a CR/CRh, the median time to first response was 3.6 months. The CR/CRh rate was 29 of 126 in patients with FLT3-ITD or FLT3-ITD/TKD and 0 of 12 in patients with FLT3-TKD only. The most common adverse reactions included skin rash, fatigue, diarrhea, elevated AST and ALT. Approximately 10% of patients required dose reductions, most commonly for diarrhea or fatigue.
It was concluded that XOSPATA® is the first and only FLT3-targeting therapy approved by the FDA, that can be used as a single agent for the treatment of adult patients who have relapsed or refractory Acute Myeloid Leukemia with a FLT3 mutation. Studies are underway evaluating XOSPATA® as maintenance therapy after allogeneic hematopoietic stem cell transplant in patients with FLT3-ITD positive AML. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm627045.htm

