SUMMARY:Prostate cancer is the most common cancer in American men, excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease. The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence.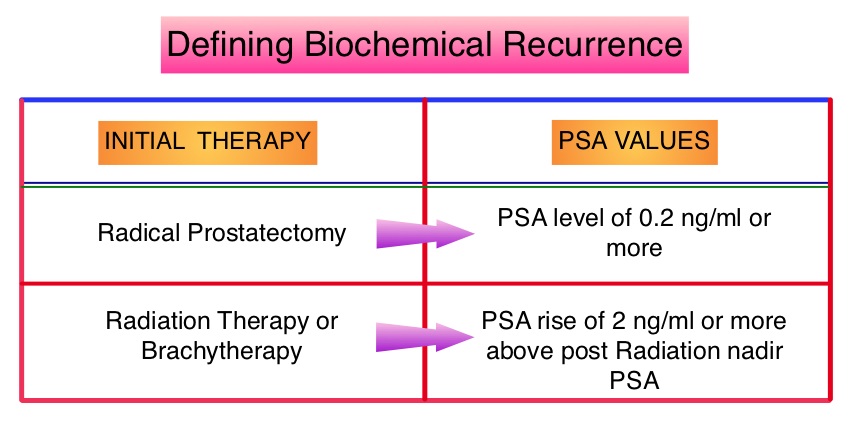 The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003)
The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003)

