SUMMARY: The U.S. Food and Drug Administration (FDA) on October 9, 2014, approved VELCADE® (Bortezomib), a proteasome inhibitor, as combination regimen, for use in previously untreated patients with Mantle Cell Lymphoma (MCL). Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. Mantle Cell Lymphomas constitute approximately 5% of all Non Hodgkin lymphomas and have a high relapse rate following dose-intensive therapies. VELCADE® was initially approved by the FDA in 2006 for the treatment of relapsed or refractory Mantle Cell Lymphoma and has a response rate of 30%.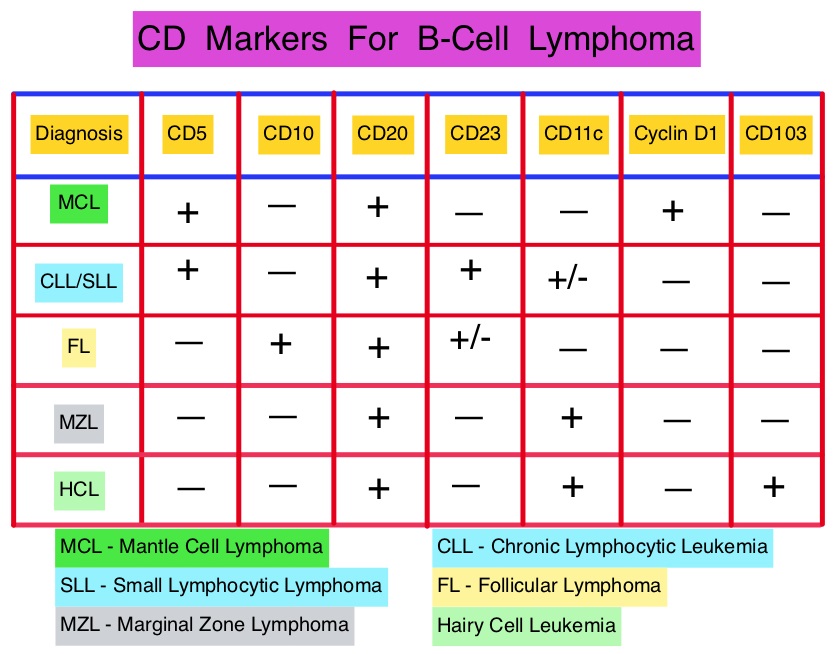 This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s
This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s

