SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. 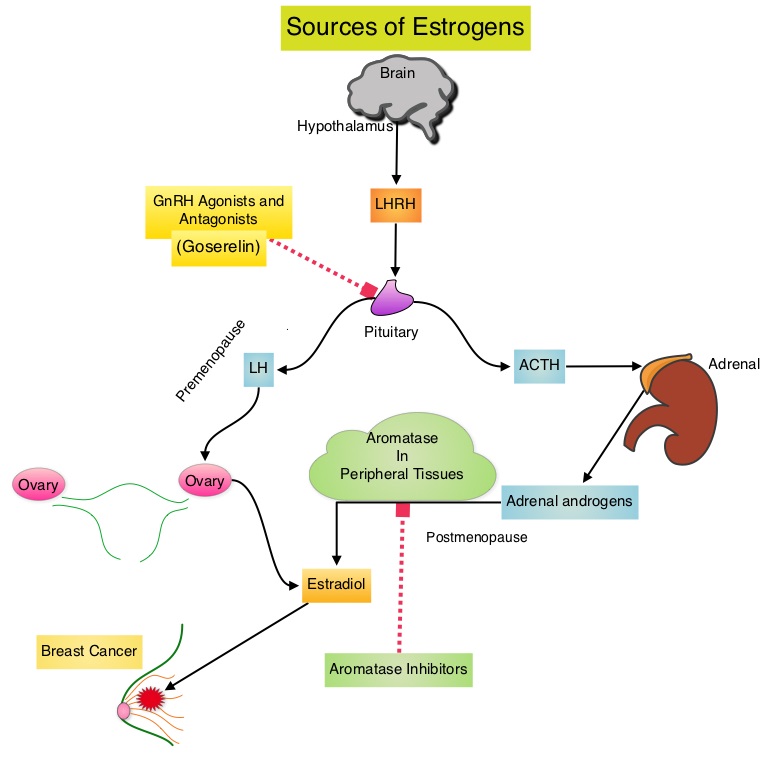 POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s

