SUMMARY: The American Cancer Society estimates that approximately 137,000 new cases of colorectal cancer will be diagnosed in the United States in 2014 and over 50,000 are expected to die of the disease. Even though colon cancer localized to the bowel is potentially curable with surgery and adjuvant chemotherapy, advanced colon cancer is often incurable. Standard chemotherapy when combined with anti EGFR (Epidermal Growth Factor Receptor) targeted monoclonal antibodies such as VECTIBIX® (Panitumumab) and ERBITUX® (Cetuximab) as well as anti VEGF agent AVASTIN® (Bevacizumab), have demonstrated improvement in Progression Free Survival and Overall Survival.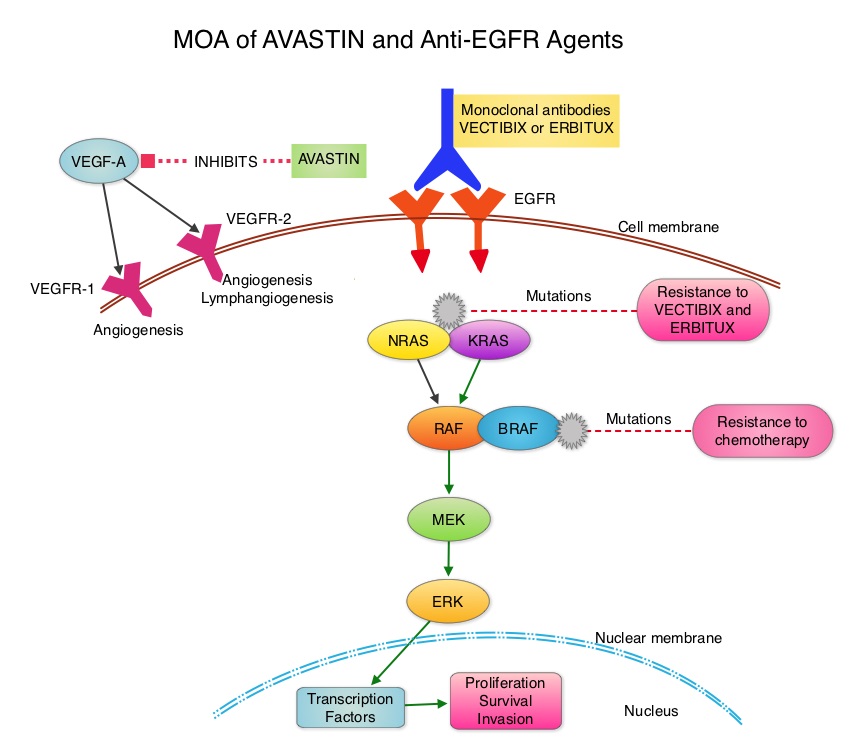 The benefit with anti EGFR agents however is only demonstrable in patients with metastatic colon cancer, whose tumors do not harbor KRAS mutations in codons 12 and 13 of exon 2 (KRAS Wild Type). It is now becoming clear that even amongst the KRAS Wild Type patient groups, about 15% to 20% have other rare mutations such as NRAS and BRAF mutations, which confer resistance to anti EGFR agents. Therefore, pan RAS (expanded RAS) testing may become relevant. To determine the optimal combination treatment regimen, this phase III intergroup trial evaluated the addition of ERBITUX® or AVASTIN® to physician’s choice of standard first line chemotherapy such as FOLFIRI or mFOLFOX6. Even though the original study included unselected metastatic colorectal cancer patients and randomization to a third arm (combination of ERBITUX® and AVASTIN®), this study was amended to include only pts with KRAS Wild Type tumors and the combination ERBITUX® and AVASTIN® arm was deleted. Patients were randomized to either ERBITUX® 400 mg/m2 week one and then 250 mg/m2, weekly or AVASTIN® 5 mg/kg every 2 weeks given along with FOLFIRI or mFOLFOX6 chemotherapy (physicians choice at the time of enrollment). The median age was 59 years and treatment groups were Chemo plus AVASTIN® (N=559) and Chemo plus ERBITUX® (N=578). Approximately 27% of the patients received FOLFIRI chemotherapy regimen and 76% received mFOLFOX6 chemotherapy regimen. Treatment was given until disease progression and median follow up was 24 months. The primary endpoint was Overall Survival. The median Overall Survival was similar in the ERBITUX® combination and the AVASTIN® combination groups (about 29 months) and so was the Progression Free Survival in both groups (about 10.5 months). The chemotherapy used with either of the antibodies had no influence on the outcomes. The toxicity profiles were different as expected, with increased incidence of Grade 3-4 rash (7% versus 0%) and diarrhea (11% versus 8%), in the ERBITUX® group and increased incidence of Grade 3-4 hypertension (7% versus 1%) and gastrointestinal events (2% versus 0.5%), in the AVASTIN® group. The authors concluded that either ERBITUX® or AVASTIN® in combination with chemotherapy have equivalent overall survival benefit, when given as first line therapy, for patients with metastatic colorectal cancer, whose tumors are KRAS Wild Type. It remains to be seen however, if pan RAS (expanded RAS) testing and other molecular studies will identify subsets of patients who will benefit from specific antibody chemotherapy combination regimens. Venook AP, Niedzwiecki D, Lenz H, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3)
The benefit with anti EGFR agents however is only demonstrable in patients with metastatic colon cancer, whose tumors do not harbor KRAS mutations in codons 12 and 13 of exon 2 (KRAS Wild Type). It is now becoming clear that even amongst the KRAS Wild Type patient groups, about 15% to 20% have other rare mutations such as NRAS and BRAF mutations, which confer resistance to anti EGFR agents. Therefore, pan RAS (expanded RAS) testing may become relevant. To determine the optimal combination treatment regimen, this phase III intergroup trial evaluated the addition of ERBITUX® or AVASTIN® to physician’s choice of standard first line chemotherapy such as FOLFIRI or mFOLFOX6. Even though the original study included unselected metastatic colorectal cancer patients and randomization to a third arm (combination of ERBITUX® and AVASTIN®), this study was amended to include only pts with KRAS Wild Type tumors and the combination ERBITUX® and AVASTIN® arm was deleted. Patients were randomized to either ERBITUX® 400 mg/m2 week one and then 250 mg/m2, weekly or AVASTIN® 5 mg/kg every 2 weeks given along with FOLFIRI or mFOLFOX6 chemotherapy (physicians choice at the time of enrollment). The median age was 59 years and treatment groups were Chemo plus AVASTIN® (N=559) and Chemo plus ERBITUX® (N=578). Approximately 27% of the patients received FOLFIRI chemotherapy regimen and 76% received mFOLFOX6 chemotherapy regimen. Treatment was given until disease progression and median follow up was 24 months. The primary endpoint was Overall Survival. The median Overall Survival was similar in the ERBITUX® combination and the AVASTIN® combination groups (about 29 months) and so was the Progression Free Survival in both groups (about 10.5 months). The chemotherapy used with either of the antibodies had no influence on the outcomes. The toxicity profiles were different as expected, with increased incidence of Grade 3-4 rash (7% versus 0%) and diarrhea (11% versus 8%), in the ERBITUX® group and increased incidence of Grade 3-4 hypertension (7% versus 1%) and gastrointestinal events (2% versus 0.5%), in the AVASTIN® group. The authors concluded that either ERBITUX® or AVASTIN® in combination with chemotherapy have equivalent overall survival benefit, when given as first line therapy, for patients with metastatic colorectal cancer, whose tumors are KRAS Wild Type. It remains to be seen however, if pan RAS (expanded RAS) testing and other molecular studies will identify subsets of patients who will benefit from specific antibody chemotherapy combination regimens. Venook AP, Niedzwiecki D, Lenz H, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3)

