SUMMARY: The FDA on July 13, 2015 approved IRESSA® (Gefitinib) for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA approved test. IRESSA was approved concurrently with a labeling expansion of the therascreen EGFR RGQ PCR Kit, a companion diagnostic test, for patient selection. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer. Epidermal Growth Factor Receptor (EGFR) is frequently overexpressed in NSCLC. In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) patients, with Adenocarcinoma histology, and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), has changed the treatment paradigm, in favor of targeted therapy, for this patient subset. GILOTRIF® is an irreversible blocker of the ErbB family, which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. IRESSA® is an oral, EGFR Tyrosine Kinase Inhibitor (TKI), which works by blocking the activity of the EGFR tyrosine kinase enzyme responsible for regulating signaling pathways, implicated in the growth and survival of cancer cells. IRESSA® was granted Orphan Drug Designation by the FDA in August 2014 for the treatment of EGFR mutation positive NSCLC.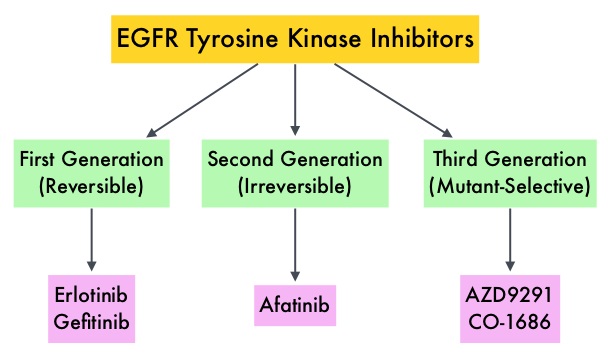
The approval of IRESSA® was based on the results of a Phase IV, single-arm, multicenter, open-label clinical study (IRESSA Follow-Up Measure or IFUM study) which included 106 treatment naïve-patients with metastatic EGFR mutation positive NSCLC who received IRESSA® 250mg PO daily. Treatment was given until disease progression or intolerable toxicity. Primary endpoint was Objective Response Rate (ORR). Secondary endpoints included Disease Control Rate (DCR), Progression Free Survival (PFS), Overall Survival (OS) and safety/tolerability. At the time of data cutoff, the investigator determined ORR was 70%, Duration of Response was 8.3 months, Disease Control Rate was 90.6%, median PFS was 9.7 months and median OS was19.2 months. This efficacy data was further supported by the IRESSA Pan-ASia Study (IPASS), a randomized phase III trial, which enrolled 1,217 treatment naïve advanced NSCLC patients with adenocarcinoma histology. Patients were randomized (1:1) to receive IRESSA® 250 mg PO daily or up to 6 cycles of combination chemotherapy with Carboplatin and Paclitaxel. The efficacy outcomes included Progression Free Survival (PFS) and Objective Response Rate (ORR). An exploratory analysis of a subset of 186 of 1217 patients (15%), who were determined to be EGFR mutation positive, had imaging studies available for evaluation (IRESSA® treated patients=88 and Carboplatin/Paclitaxel treated patients=98). The median PFS in the IRESSA® treated group was 10.9 months compared to 7.4 months for the Carboplatin/Paclitaxel treated patients (HR=0.54). The ORR was 67% with a Duration of Response (DoR) of 9.6 months for IRESSA® treated patients versus 41%, with a DoR of 5.5 months for Carboplatin/Paclitaxel treated patients. The most commonly reported adverse events for IRESSA® were diarrhea and skin toxicities including rash, acne, dry skin and pruritus. It was concluded that EGFR mutations are the strongest predictive biomarker for Progression Free Survival and tumor response to first line treatment with IRESSA®. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Douillard J-Y, Ostoros G, Cobo M, et al. Br J Cancer. 2014;110:55–62

