SUMMARY: The FDA on December 11, 2015 granted accelerated approval to Alectinib (ALECENSA®) for the treatment of patients with Anaplastic Lymphoma Kinase (ALK)-positive metastatic Non Small Cell Lung Cancer (NSCLC), who have progressed on or are intolerant to XALKORI® (Crizotinib). Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 25% are Squamous cell carcinomas, 40% are Adenocarcinomas and 10% are Large cell carcinomas. The discovery of rearrangements of the Anaplastic Lymphoma Kinase (ALK) gene in some patients with advanced NSCLC and adenocarcinoma histology, led to the development of agents such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib), with promising results. It has become clear that appropriate, molecularly targeted therapy for tumors with a molecular abnormality, results in the best outcomes. According to the US Lung Cancer Mutation Consortium (LCMC), two thirds of patients with advanced adenocarcinoma of the lung, have a molecular driver abnormality. The most common oncogenic drivers in patients with advanced adenocarcinoma of the lung are, KRAS in 25%, EGFR in 21% and ALK in 8% as well as other mutations in BRAF, HER2, AKT1 and fusions involving RET and ROS oncogenes. These mutations are mutually exclusive and the presence of two simultaneous mutations, are rare.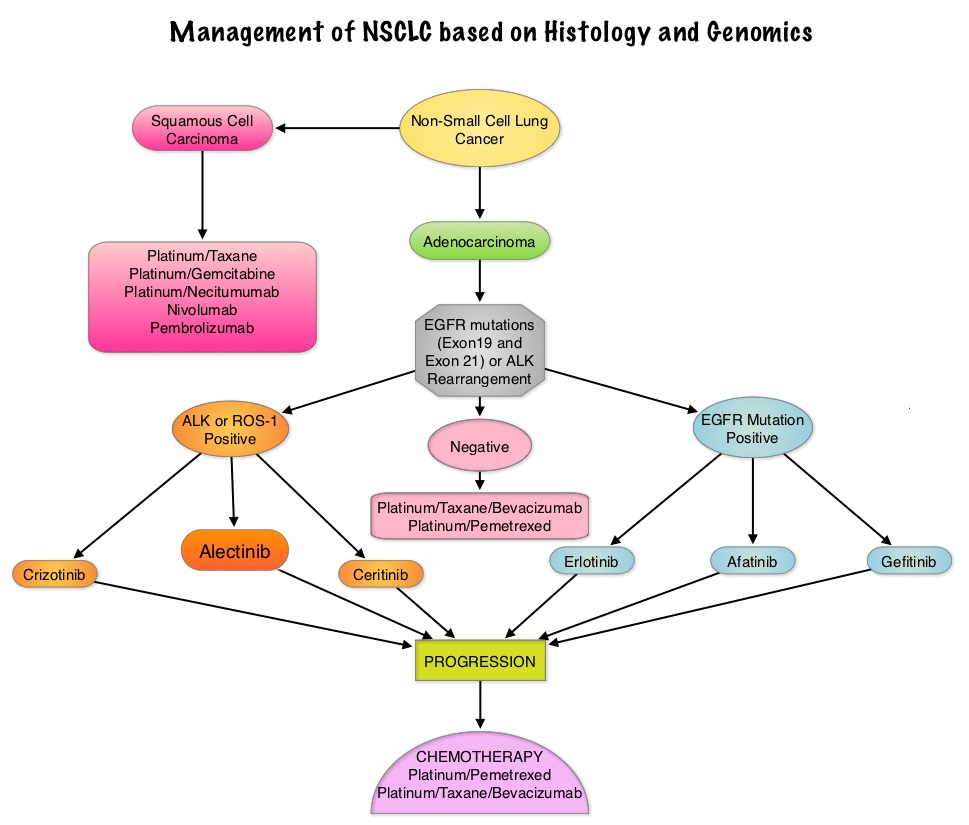
The approval of ALECENSA®) was based on two multicenter, single arm, open label, clinical trials (Study 1 and 2) in which enrolled patients received ALECENSA® 600 mg twice daily. The primary end point was Objective Response Rate (ORR). Secondary end points included Duration of Response (DoR), Objective Response Rate in the Central Nervous System (CNS) in those with measurable lesions in the CNS, and CNS Duration of Response. In Study 1 (N=87), the ORR was 38% and the Duration of Response was 7.5 months. In Study 2 (N=138), the ORR was 44% and the Duration of Response was 11.2 months. In a pooled analysis of patients from both Study 1 and Study 2 with measurable CNS lesions, the CNS Objective Response Rate was 61% and the median CNS Duration of Response was 9.1 months. The most common grade 1-2 adverse events were fatigue, constipation, edema, myalgia, anemia and elevation in liver function tests. The most common but rare grade 3-4 adverse reaction was dyspnea. It should be noted that ZYKADIA® (Ceritinib) is already approved for a similar patient population. A global phase III trial comparing ALECENSA® with XALKORI® as first line treatment, is presently underway.
A phase II, open-label, multicenter study of the ALK inhibitor alectinib in an ALK+ non-small-cell lung cancer (NSCLC) U.S./Canadian population who had progressed on crizotinib (NP28761). Gandhi L, Shaw A, Gadgeel SM, et al. J Clin Oncol 33, 2015 (suppl; abstr 8019)
Alectinib in Crizotinib-Refractory ALK-Rearranged Non–Small-Cell Lung Cancer: A Phase II Global Study. Ou SI, Ahn JS, Petris LD, et al. Published online before print November 23, 2015, doi: 10.1200/JCO.2015.63.9443

