SUMMARY: The FDA on September 4, 2020, granted accelerated approval to GAVRETO® (Pralsetinib) for adult patients with metastatic RET fusion-positive Non Small Cell Lung Cancer (NSCLC), as detected by an FDA approved test. The FDA also approved the Oncomine Dx Target (ODxT) Test as a companion diagnostic for GAVRETO®. Lung cancer is the second most common cancer in both men and women 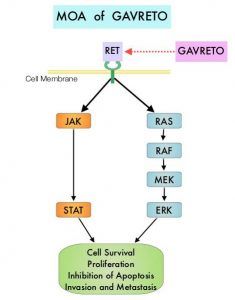 and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers.
and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers.
In addition to the well characterized gene fusions involving ALK and ROS1 in NSCLC, genetic alterations involving other kinases including EGFR, BRAF, RET, NTRK, are all additional established targetable drivers. These genetic alterations are generally mutually exclusive, with no more than one predominant driver in any given cancer. The hallmark of all of these genetic alterations is oncogene addiction, in which cancers are driven primarily, or even exclusively, by aberrant oncogene signaling, and are highly susceptible to small molecule inhibitors.
RET kinase is a transmembrane Receptor Tyrosine Kinase and plays an important role during the development and maintenance of a variety of tissues, including neural and genitourinary tissues. RET signaling activates downstream pathways such as JAK/STAT3 and RAS/RAF/MEK/ERK and leads to cellular proliferation, survival, invasion, and metastasis. Oncogenic alterations to the RET proto-oncogene results in uncontrolled cell growth and enhanced tumor invasiveness. RET alterations include RET rearrangements, leading to RET fusions, and activating point mutations occurring across multiple tumor types. RET fusions have been identified in approximately 2% of NSCLCs, 10-20% of non-medullary thyroid cancers. Activating RET point mutations account for approximately 60% of sporadic Medullary Thyroid Cancers (MTC) and more than 90% of inherited MTCs. Other cancers with documented RET alterations include colorectal, breast, and several hematologic malignancies.
GAVRETO® is an oral, highly potent, selective RET kinase inhibitor targeting oncogenic RET alterations, including fusions and mutations, regardless of the tissue of origin. The efficacy of GAVRETO® was investigated in a multicenter, open-label, multi-cohort, Phase I/II basket clinical trial (ARROW), in patients with tumors showing RET alterations. Identification of RET gene alterations was prospectively determined in local laboratories using either Next Generation Sequencing (NGS), Fluorescence In Situ Hybridization (FISH), or other tests. (In a basket trial, tumors with different histologies and single biomarker are placed in different baskets and receive a single treatment). The main efficacy outcome measures were Overall Response Rate (ORR) and response duration, as determined by a blinded Independent Review Committee, using RECIST criteria.
The efficacy for RET fusion-positive NSCLC was evaluated in 87 patients previously treated with platinum-based chemotherapy. Patients received GAVRETO® 400 mg orally once daily. The ORR was 57%, with a Complete Response (CR) rate of 5.7% and 80% of responding patients had responses lasting 6 months or longer. The median Duration of Response was not reached. Efficacy was also evaluated in 27 patients who never received systemic treatment and the ORR in this patient group was 70% with 11% CR rate and 58% of responding patients had responses lasting 6 months or longer. The most common adverse reactions (25% or more) were fatigue, constipation, musculoskeletal pain and hypertension.
It was concluded that patients treated with GAVRETO® had a rapid, potent, and durable clinical response, in patients with advanced RET fusion positive NSCLC, regardless of RET fusion partner, presence of brain metastases, or prior therapies.
Gainor JF, Curigliano G, Kim D-W, et al. DOI: 10.1200/JCO.2020.38.15_suppl.9515 Journal of Clinical Oncology 38, no. 15_suppl (May 20, 2020) 9515-9515.

