SUMMARY: The FDA on August 16, 2018, granted accelerated approval to OPDIVO® (Nivolumab), for patients with metastatic Small Cell Lung Cancer (SCLC) with progression after platinum-based chemotherapy and at least one other line of therapy. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Small cell lung cancer (SCLC) accounts for approximately 13-15% of all lung cancers and is aggressive. Patients with SCLC are often treated with platinum based chemotherapy as first-line treatment and the tumor response rates are as high as 60-80%. However, only 20% of patients with Limited Stage SCLC are cured and majority of the patients relapse within months of completing initial therapy. The only FDA-approved agent for recurrent or progressive SCLC (second-line treatment) is HYCAMTIN® (Topotecan) and there is presently no standard therapy, after failure on second-line therapy. The 5 year survival rate for Extensive Stage SCLC is less than 5%, with a median survival of 9 to 10 months from the time of diagnosis.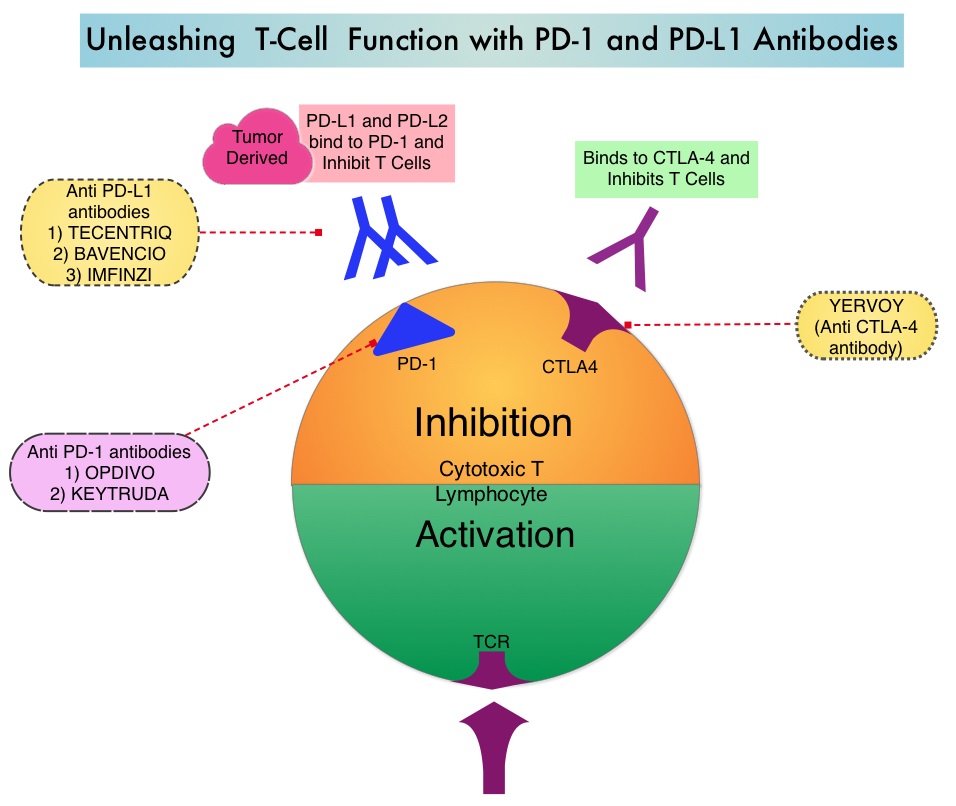
OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor (Checkpoint proteins) and blocks its interaction with PD-L1 and PD-L2. Blocking the Immune checkpoint proteins unleashes the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The present FDA approval for OPDIVO® was based on the results of phase I/II CheckMate-032 trial, which is a multicenter, open-label, ongoing study. This study included 245 patients with SCLC who experienced disease progression after platinum-based chemotherapy. Efficacy data was submitted by the investigators to the FDA from 109 patients who received OPDIVO® after disease progression on platinum-based chemotherapy and at least one other prior line of therapy, to support this present indication. Patients received OPDIVO® 3 mg/kg IV every 2 weeks until disease progression or unacceptable toxicity. The first tumor assessments were conducted 6 weeks after the first dose and were continued every 6 weeks for the first 24 weeks and every 12 weeks thereafter. The Primary endpoint of the study was Objective Response Rate (ORR). Secondary outcome measures included Overall Survival (OS), Progression Free Survival (PFS), Duration of Response (DOR), and the occurrence of treatment-related Adverse Events (AEs) leading to treatment discontinuation.
The results from a blinded, independent central review showed that the ORR was 12% and among the responders, the median Duration of Response was 17.9 months. The responses were durable for 6 months or longer in 77%, 12 months or longer in 62%, and 18 months or longer in 39% of the responding patients. These treatment responses were noted regardless of PD-L1 expression. OPDIVO® was granted accelerated approval for this indication on the basis of Overall Response Rate and Duration of Response, and further proof of benefit in confirmatory trials may be required for full approval.
It was concluded that OPDIVO® is the first new agent approved in nearly 20 years for Small Cell Lung Cancer, and is the first checkpoint inhibitor approved for this patient group. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617370.htm

