SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality. Heparin Induced Thrombocytopenia (HIT) is a prothrombotic disorder caused by antibodies to complexes of Platelet Factor 4 (PF4), a protein present in the platelet alpha granules and heparin and the incidence of HIT varies from 3-5% in patients treated with unfractionated heparin. The frequency of thromboemboli in HIT patients is 30-50% and women diagnosed with HIT are at a 1.7 times greater risk for thrombotic manifestations than men.
There are two types of HIT. Type 1 HIT is a non-immune disorder that results from the direct effect of heparin on platelet activation and manifests within the first 2 days after heparin exposure to heparin, and the platelet count normalizes with continued heparin therapy. Type 2 HIT however is an immune-mediated disorder that typically occurs 4-10 days after exposure to heparin and can result in life threatening thrombotic complications. Patients with HIT more often experience thrombotic events such as Deep Venous Thrombosis, Pulmonary Embolism and sometimes Arterial thrombosis rather than bleeding episodes. The 4 T’s that raise clinical suspicion for HIT include Thrombocytopenia, Timing of thrombocytopenia, Thrombosis and ruling out oTher causes of thrombocytopenia. Once a diagnosis of HIT is established, all heparin products should be stopped and alternative anticoagulants should be considered such as ARGATROBAN®, REFLUDAN® (Lepirudin), ANGIOMAXreg; (Bivalirudin) and ARIXTRA® (Fondaparinux). Warfarin may cause microthrombosis in patients with HIT and should be avoided and should be started only after the platelet count exceeds 150 x 109/L. IVC filters should be avoided as well.
The currently approved therapies for the treatment of HIT however are parenteral preparations and require laboratory coagulation monitoring. XARELTO® is a direct oral anti-Xa inhibitor and is presently approved by the FDA for the prevention and treatment of Deep Vein Thrombosis and Pulmonary Embolism as well as prevention of thromboembolic events in patients with Atrial Fibrillation. XARELTO® could be an ideal agent for patients with HIT, as it can be administered orally at a fixed dose and does not require routine coagulation monitoring.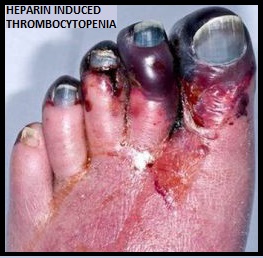
The purpose of this study was to determine the safety and efficacy of XARELTO® in patients suspected or confirmed to have HIT. The authors in this multicenter, single-arm, prospective cohort study, reviewed the data of 22 consecutive adults with suspected or confirmed HIT. Patients received XARELTO® 15 mg PO BID until a local HIT assay result was available. Patients with a positive local assay result continued XARELTO® 15 mg PO BID until platelet recovery (or until day 21 if they had acute thrombosis at the time of entry into the study). The dose of XARELTO® was then changed to 20 mg PO daily, until day 30. This study was slated to enroll 200 patients but the study was terminated early after 22 patients were enrolled, because of difficulty in recruitment.
It was noted that the incidence of new, symptomatic, objectively confirmed, venous or arterial thromboembolism at 30 days in the HIT positive group (Primary endpoint), was 4.5% and one HIT-positive patient required limb amputation despite platelet recovery. Nine out of 10 HIT-positive patients with thrombocytopenia had platelet recovery.
It was concluded that based on this small study, XARELTO® was effective for treating patients with confirmed HIT, and also facilitated platelet recovery. This first prospective study of XARELTO® in HIT patients has a limited number of patients and the 22 patients in this study were enrolled over a 2.5 year period, which demonstrated the difficulty in enrolling patients in this study. Nonetheless, it is unlikely that larger studies will be designed to compare XARELTO® to one of the parenteral preparations. Based on the available data, XARELTO® may fulfill an unmet need for the management of patients with Heparin Induced Thrombocytopenia. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. Linkins LA, Warkentin TE, Pai M, et al. J Thromb Haemost 2016;14:1206-1210.

