SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis (DVT)/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality, after myocardial infarction and stroke. Ambulatory cancer patients initiating chemotherapy are at varying risk for Venous Thromboembolism (VTE), which in turn can have a substantial effect on health care costs, with negative impact on quality of life.
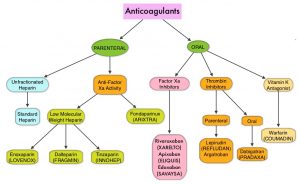
Approximately 20% of cancer patients develop VTE and about 20% of all VTE cases occur in patients with cancer. There is a two-fold increase in the risk of recurrent thrombosis in patients with cancer, compared with those without cancer, and patients with cancer and VTE are at a markedly increased risk for morbidity and mortality. The high risk of recurrent VTE, as well as bleeding in this patient group, makes anticoagulant treatment challenging. Treatment with parenteral Low Molecular Weight Heparin (LMWH) preparations is often recommended for this patient group, based on efficacy data. LMWH activates antithrombin, which in turn accelerates the inactivation of coagulation enzymes thrombin (Factor IIa), Factor Xa and Factor IXa. Parenteral LMWH however can be inconvenient and expensive, leading to premature discontinuation of treatment.
Direct Oral Anticoagulant agents have been proven to be as effective as COUMADIN® (Warfarin), a Vitamin K antagonist, for the treatment of VTE, and are associated with less frequent and less severe bleeding, and fewer drug interactions. The Direct Oral AntiCoagulants (DOACs) include PRADAXA® (Dabigatran), which is a direct Thrombin inhibitor and XARELTO® (Rivaroxaban), ELIQUIS® (Apixaban), SAVAYSA® (Edoxaban), BEVYXXA® (Betrixaban), which are Factor Xa inhibitors. Compared to COUMADIN®, the New Oral Anticoagulants have a rapid onset of action, wider therapeutic window, shorter half-lives (7-14 hours in healthy individuals), require no laboratory monitoring and have a fixed dosing schedule.
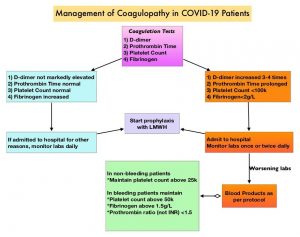
Patients with cancer are often found to have distal DVTs. In patients with isolated distal DVT, the best treatment strategy remains unclear and evidence is lacking for the optimal duration of anticoagulation therapy. Further, prolonged anticoagulation therapy, beneficial as it may be, could increase the risk of bleeding.
The ONCO DVT study is a multicenter, open-label, adjudicator-blinded, randomized, clinical trial, conducted at 60 institutions in Japan. This study included 601 cancer patients with isolated distal DVT, who were randomly assigned in a 1:1 ratio, to receive either Edoxaban (SAVAYSA®) for 12 months (N=296) or 3 months (N=305). Edoxaban was given at a dose of 60 mg orally once daily but it was dose-reduced to 30 mg in 75% of patients due to either creatinine clearance of 30-50 mL/min or a body weight of 60 kg or less, or due to concomitant treatment with potent P-glycoprotein inhibitors. The researchers hypothesized that 12-month Edoxaban treatment was superior to a 3-month Edoxaban treatment for reducing thrombotic events, in cancer patients with isolated distal DVT. Eligible patients had active cancer and newly diagnosed isolated distal DVT confirmed by ultrasonography. The mean age was 71 years and 72% of the patients were women. The most common type of cancer was gynecologic cancer (27%), followed by lung cancer (11%), colon cancer (10%) and pancreatic cancer (8%). The most common reason for conducting ultrasonography was due to a high-risk status with elevated D-dimer levels (38%), followed by elevated D-dimer levels before surgery (24%) and suspected DVT based on the symptoms (20%). Patients were excluded from this study if they were on anticoagulation therapy at the time of the diagnosis, if they had pulmonary embolism, or if they were expected to have a life prognosis of 3 months or less by the treating physicians. The Primary endpoint was symptomatic recurrent Venous ThromboEmbolism (VTE) or VTE-related death at 12 months. The Secondary endpoint was major bleeding at 12 months, according to the criteria of the International Society on Thrombosis and Hemostasis.
The Primary endpoint of a symptomatic recurrent VTE event or VTE-related death occurred in 1% of patients in the 12-month Edoxaban group and in 7.2% of patients in the 3-month Edoxaban group (odds ratio, 0.13). The Secondary endpoint of major bleeding occurred in 9.5% of patients in the 12-month edoxaban group and in 7.2%of patients in the 3-month edoxaban group (Odds Ratio=1.34), and this was not statistically significant. There were no differences noted in prespecified subgroup analyses, stratified by age, body weight and renal function.
It was concluded that in cancer patients with symptomatic or asymptomatic isolated distal DVT, anticoagulation with Edoxaban for 12 months was superior to 3 months with respect to symptomatic recurrent VTE or VTE-related death, without increasing the risk of bleeding. The authors added that this is the first and only randomized clinical trial to show the superiority of longer duration, over shorter duration anticoagulation therapy, for reducing thrombotic events in cancer patients with isolated distal DVT. Since this study only included Japanese, it is unclear if this data would be applicable to people of other races and ethnicities.
Edoxaban for 12 Months Versus 3 Months in Cancer Patients with Isolated Distal Deep Vein Thrombosis (ONCO DVT study): An Open-label, Multicenter, Randomized Clinical Trial. Yamashita Y, Morimoto T, Muraoka N, et al., on behalf of the ONCO DVT Study Investigators. Originally published 28 Aug 2023. https://doi.org/10.1161/CIRCULATIONAHA.123.066360 Circulation. 2023;0