SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis (DVT)/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality, after myocardial infarction and stroke. Ambulatory cancer patients initiating chemotherapy are at varying risk for Venous Thromboembolism (VTE), which in turn can have a substantial effect on health care costs, with negative impact on quality of life. Approximately 20% of cancer patients develop VTE and there is a two-fold increase in the risk of recurrent thrombosis in patients with cancer, compared with those without cancer. The benefit of thromboprophylaxis in this patient population however is uncertain. This is because previously published randomized trials included cancer patients both at both low and high risk for VTE.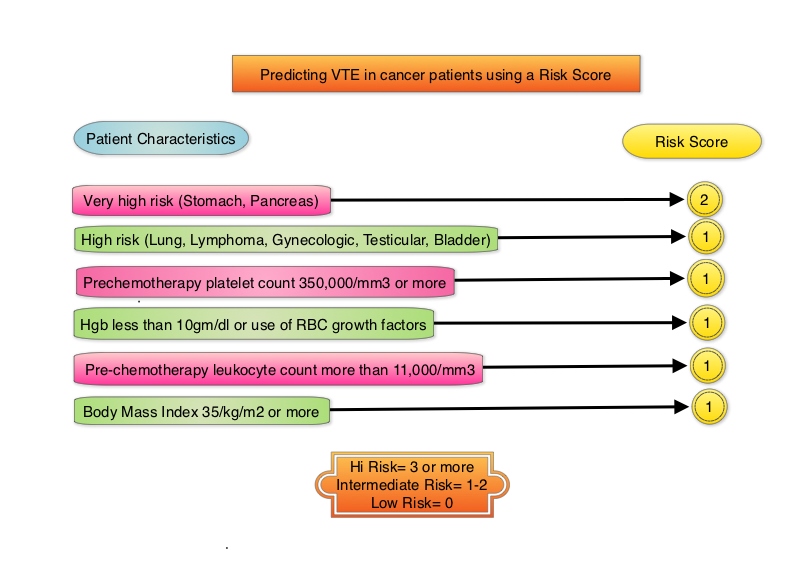
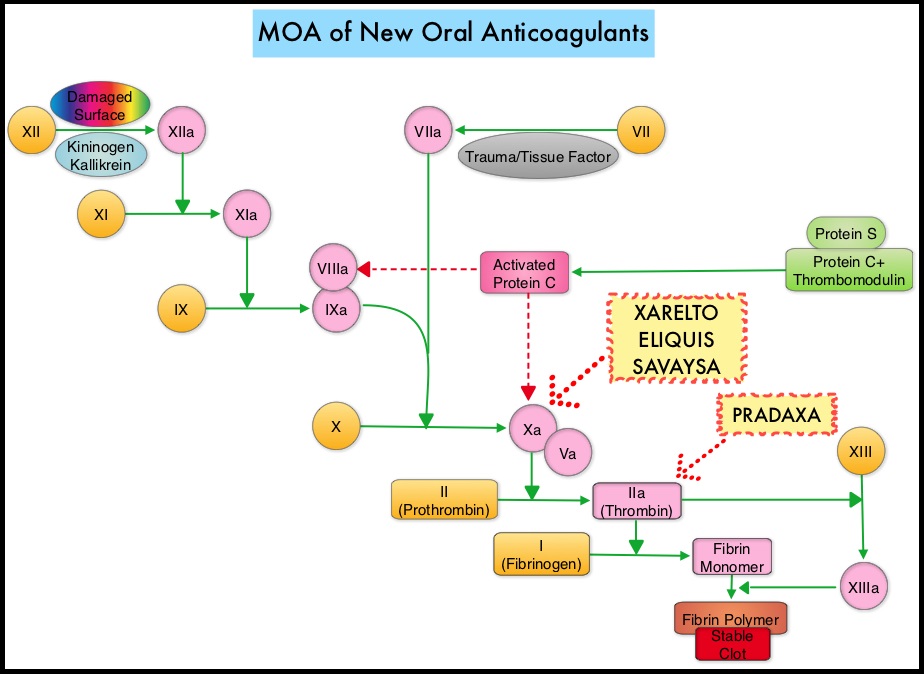
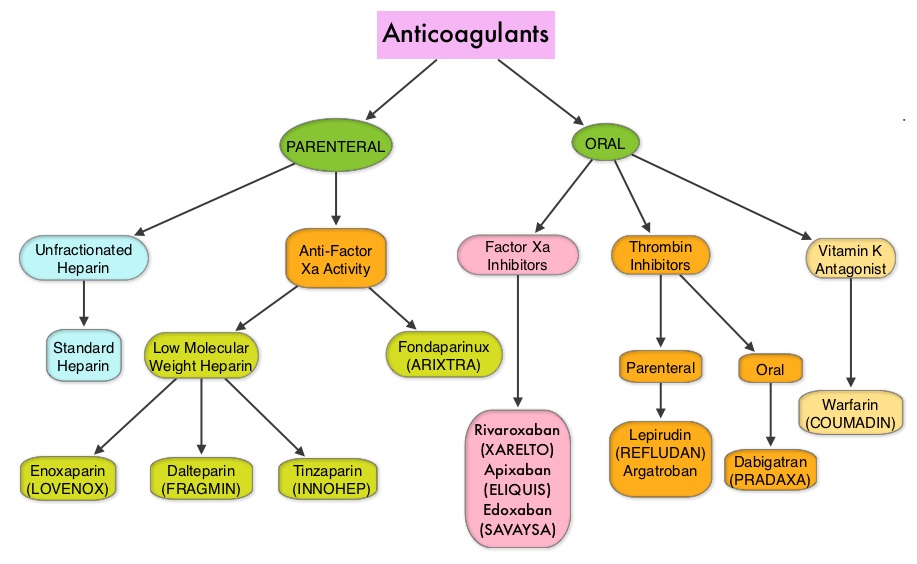
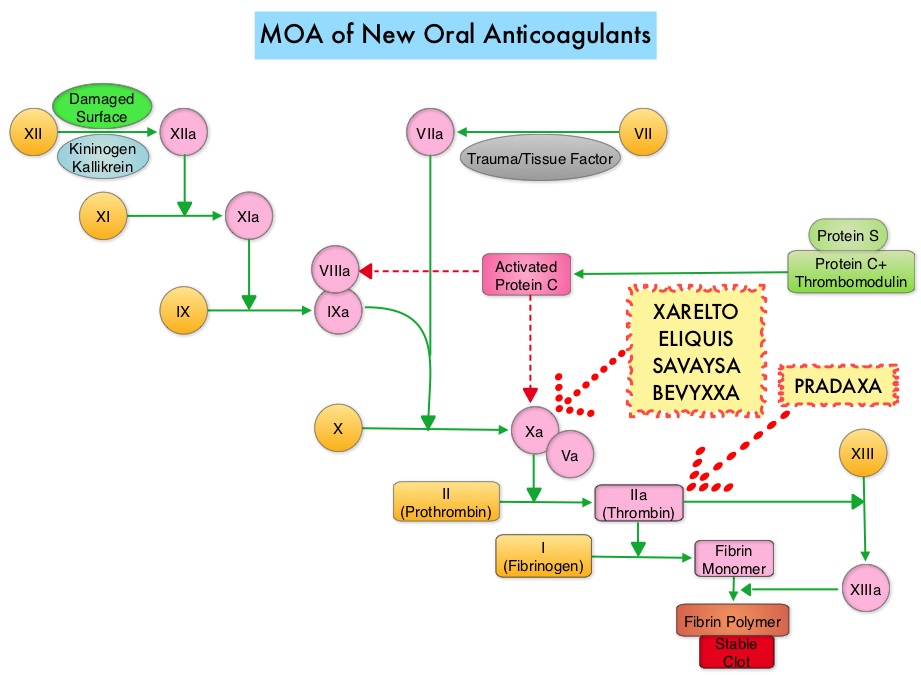
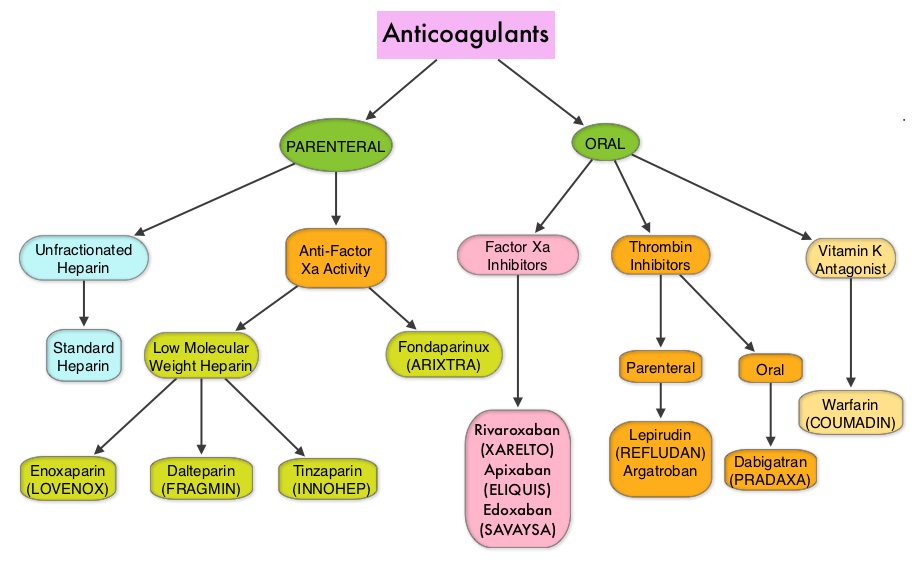
Khorana score is a validated risk tool which helps to identify patients at increased risk for VTE. Several studies have suggested benefit from thromboprophylaxis in patients with a score of 3 or higher, whereas the benefit of thromboprophylaxis in patients with a score of 2 is unclear, although there is a substantial risk of VTE in this group as well. The current recommendations are treatment with parenteral Low Molecular Weight Heparin (LMWH) preparations for at least 6 months or probably longer, as long as the cancer is active. This however can be inconvenient and expensive, leading to premature discontinuation of treatment. LMWH accelerates the inhibition by Antithrombin of activated Factor X, in the conversion of Prothrombin to Thrombin. Direct Oral AntiCoagulants (DOACs) have been proven to be noninferior to COUMADIN® (Warfarin), a Vitamin K antagonist, for the treatment of acute VTE, and are associated with less frequent and less severe bleeding and fewer drug interactions. The Direct Oral AntiCoagulants (DOACs) include PRADAXA® (Dabigatran), which is a direct Thrombin inhibitor and XARELTO® (Rivaroxaban), ELIQUIS® (Apixaban), SAVAYSA® (Edoxaban), BEVYXXA® (Betrixaban) which are Factor Xa inhibitors. Compared to COUMADIN® , the New Oral Anticoagulants have a rapid onset of action, wider therapeutic window, shorter half-lives (7-14 hours in healthy individuals), no laboratory monitoring and fixed dosing schedule.
The AVERT (Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients ) trial is a randomized, placebo-controlled, double-blind clinical trial which evaluated the efficacy and safety of apixaban (2.5 mg twice daily) for thromboprophylaxis in ambulatory patients with cancer who were at intermediate-to-high risk for venous thromboembolism (Khorana score 2 or more). Eligible patients (N=574) were randomized in a 1:1 ratio to receive apixaban or placebo and 563 patients were included in the modified intention-to-treat analysis. The first dose of apixaban or placebo was administered within 24 hours after the initiation of chemotherapy. The mean patient age was 61 years, and the common types of primary malignancies were gynecologic (25.8%), lymphoma (25.3%), and pancreatic (13.6%). Eligible patients included those who had a newly diagnosed cancer or progression of known cancer after complete or partial remission and who were initiating a new course of chemotherapy with a minimum treatment intent of 3 months. Inclusion required a Khorana score of 2 or higher. Exclusion criteria included hepatic disease associated with coagulopathy, platelet count of less than 50,000 per cubic millimeter, acute leukemia, myeloproliferative neoplasm, planned stem-cell transplantation and GFR of less than 30 ml/min. The Primary efficacy outcome was objectively documented venous thromboembolism over a follow-up period of 180 days. The main Safety outcome was a major bleeding episode.
Venous thromboembolism occurred in 4.2% in the apixaban group and 10.2% in the placebo group (HR=0.41; P<0.001). This benefit and was predominantly driven by a lower rate of pulmonary embolism in the apixaban group than in the placebo group. The rate of major bleeding was significantly higher with apixaban than with placebo in the modified intention-to-treat analysis (3.5% versus 1.8%, respectively; HR=2.00), but the rate however was not significantly higher with apixaban than with placebo in the analysis of outcomes during the treatment period (2.1% versus 1.1%, respectively; HR=1.89). There was no significant difference in the Overall Survival between the treatment groups and the authors attributed this to trial design and the fact that most of the patients had advanced cancer, which was the most common cause of death.
It was concluded that thromboprophylaxis with apixaban at a dose of 2.5 mg twice daily resulted in a significantly lower risk of venous thromboembolism when compared to placebo, among ambulatory cancer patients who were initiating chemotherapy, and had an intermediate to high risk of venous thromboembolism. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. Carrier M, Abou-Nassar K, Mallick R, et al. for the AVERT Investigators. N Engl J Med 2019;380:711-719