SUMMARY: It is estimated that up to 30% of patients with nonvalvular atrial fibrillation may receive antiplatelet agents along with oral anticoagulants, due to comorbid cardiovascular conditions. The concomitant use of Vitamin K Antagonist (VKA) such as Warfarin along with antiplatelet agents, has in previously published studies, shown to increase the risk of bleeding, compared with VKAs alone.
Direct Oral AntiCoagulants (DOACs) are often prescribed for thromboembolic events. This class of anticoagulants, have a rapid onset and offset of action, short half-life, predictable anticoagulant effects, no laboratory monitoring and fixed dosing schedule. The half-life of these agents can however be prolonged in those with renal insufficiency and may be unsafe and DOACs are ineffective in patients with mechanical heart valves. Direct Oral AntiCoagulants have a favorable efficacy and safety profile, compared with Vitamin K Antagonists (VKAs) and are increasingly being used for ischemic stroke prevention among patients with nonvalvular atrial fibrillation. In several clinical studies, DOACs have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to Vitamin K Antagonist (VKA) such as Warfarin. Meta-analysis of randomized controlled trials (RCTs) assessing the efficacy of DOACs along with AcetylSalicylic Acid (ASA), in nonvalvular atrial fibrillation has shown similar risk of major bleeding but a decreased risk of intracranial hemorrhage, when compared with VKAs plus ASA. Some of the studies included in this meta-analysis however had methodological limitations.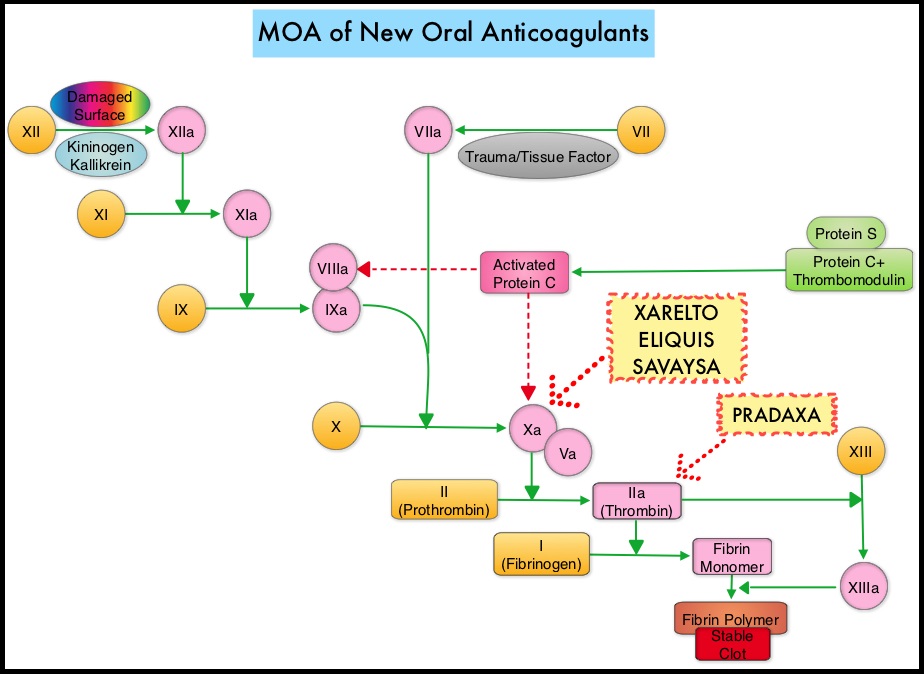
In order to address this clinically important safety issue, the authors conducted this population-based study to compare the incidence of intracranial hemorrhage, gastrointestinal bleeding, and other major bleeding between concomitant DOAC/antiplatelet use and concomitant VKA/antiplatelet use, in patients with nonvalvular atrial fibrillation. This study was conducted among a cohort of patients with newly diagnosed nonvalvular atrial fibrillation, between January 2011 and March 2014, using computerized health care databases from Quebec. Of the 14, 407 patients included in this study, 5301 patients initiated concomitant DOAC/antiplatelet use, while 9106 patients initiated concomitant VKA/antiplatelet use. DOACs included PRADAXA® (Dabigatran), XARELTO® (Rivaroxaban), or ELIQUIS® (Apixaban) and antiplatelet agents included ASA (Aspirin), Dipyridamole, PLAVIX® (Clopidogrel), EFFIENT® (Prasugrel), or BRILINTA® (Ticagrelor). Three separate analyses were conducted for intracranial hemorrhage, gastrointestinal bleeding, and other major bleeding. The median follow up was 1.6 months which was primarily driven by discontinuation of antiplatelet therapy.
It was noted that concomitant DOAC/antiplatelet therapy was associated with a similar risk of gastrointestinal bleeding (HR 1.08) but with a decreased risk of intracranial hemorrhage (HR 0.46) and other major bleeding (HR 0.68), compared with concomitant VKA/antiplatelet therapy.
The authors concluded that based on the results of this population-based study, compared with concomitant Vitamin K Antagonist /antiplatelet use, concomitant Direct Oral AntiCoagulants/antiplatelet use was associated with a similar risk of gastrointestinal bleeding, but a lower risk of intracranial hemorrhage and other major bleeding. These findings could provide guidance to physicians and help in decision making, for patients requiring concomitant treatment with oral anticoagulants and antiplatelets. Concomitant Use of Direct Oral Anticoagulants with Antiplatelet Agents and the Risk of Major Bleeding in Patients with Nonvalvular Atrial Fibrillation. Douros A, Renoux C, Yin H, et al. The American Journal of Medicine 2019; 132:191-199

