SUMMARY: The Centers for Disease Control and Prevention estimates that in the US, there are more than 16,000 cases of Human PapillomaVirus (HPV)-positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) per year and there has been a significant increase in incidence during the past several decades. They represent approximately 70% of all OPSCC in the United States and Canada. Patients with HPV-positive OPSCC tend to be younger males, who are former smokers or nonsmokers, with risk factors for exposure to High Risk HPV (HR-HPV). The HPV-positive primary Squamous Cell Carcinoma tend to be smaller in size, with early nodal metastases, and these patients have a better prognosis compared with patients with HPV-negative Head and Neck Squamous Cell Carcinoma (HNSCC), when treated similarly. Expression of tumor suppressor protein, known as p16, is highly correlated with infection with HPV in HNSCC. Accurate HPV assessment in head and neck cancers is becoming important as it significantly impacts clinical management.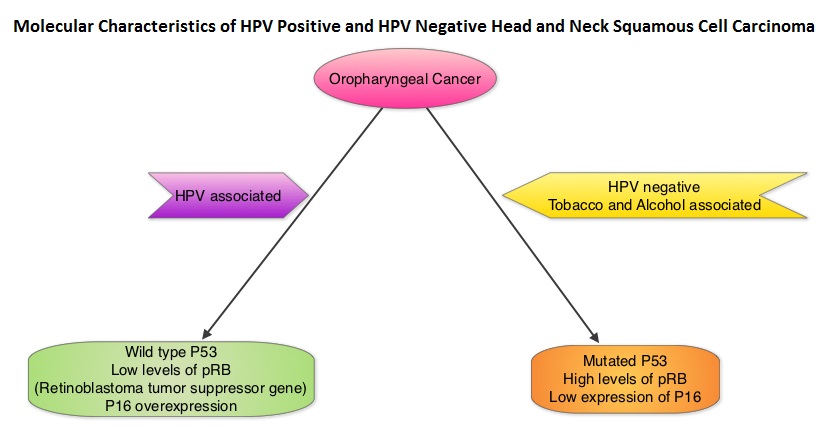
There is currently no consensus on when to test oropharyngeal squamous cell carcinomas for HPV/p16, and which tests to choose. The College of American Pathologists convened a panel of experts and following review of evidence from over 400 peer reviewed articles, came up with the following Guideline. This guideline is recommended for all new Oropharyngeal Squamous cell carcinoma patients, but not routinely recommended for other head and neck carcinomas.
Summary of Guideline Statements
1) High-Risk (HR) HPV testing should be performed on all patients with newly diagnosed OPSCC, including all histologic subtypes and may be performed on the primary tumor or a regional lymph node metastasis when the clinical findings are consistent with an oropharyngeal primary. This test should not be routinely performed on nonsquamous carcinomas of the oropharynx, or nonoropharyngeal primary carcinomas of the head and neck.
2) For oropharyngeal tissue specimens (ie, noncytology), HR-HPV testing should be performed by surrogate marker p16 ImmunoHistoChemistry (IHC). Additional HPV-specific testing may be done at the discretion of the pathologist and/or treating clinician, or in the context of a clinical trial.
3) HR-HPV testing by surrogate marker p16 IHC should be routinely performed on patients with metastatic Squamous Cell Carcinoma of unknown primary in a cervical upper or mid jugular chain lymph node. An explanatory note on the significance of a positive HPV result is recommended.
4) HR-HPV testing should be performed on head and neck FNA (Fine Needle Aspiration) Squamous Cell Carcinoma samples from all patients with known OPSCC not previously tested for HR-HPV, with suspected OPSCC, or with metastatic SCC of unknown primary.
5) Pathologists should report p16 IHC positivity as a surrogate for HR-HPV in tissue specimens (ie, noncytology) when there is at least 70% nuclear and cytoplasmic expression with at least moderate to strong intensity.
6) Pathologists should not routinely perform low-risk HPV testing on patients with head and neck carcinomas.
7) For HPV-positive/p16 cases, tumor grade (or differentiation status) is not recommended.
8) HR-HPV testing strategy should not be altered based on patient smoking history.
9) Pathologists should report primary OPSCCs that test positive for HR-HPV or its surrogate marker p16 as HPV positive and/or p16 positive
Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Lewis JS, Beadle B, Bishop JA, et al. https://doi.org/10.5858/arpa.2017-0286-CP

