SUMMARY: The FDA on August 5, 2016, granted accelerated approval to KEYTRUDA® (Pembrolizumab) for the treatment of patients with recurrent or metastatic Head and Neck Squamous Cell Carcinoma (HNSCC), with disease progression on or after Platinum containing chemotherapy. The American Cancer Society estimates that 61,760 people will be diagnosed with Head and Neck cancer in 2016 and 13,190 patients will die of the disease. Patients with recurrent/metastatic Squamous Cell Carcinoma of the Head and Neck have a poor prognosis with a median Overall Survival (OS) of about 13 months with first line therapy and about 6 months or less with later lines of therapy. 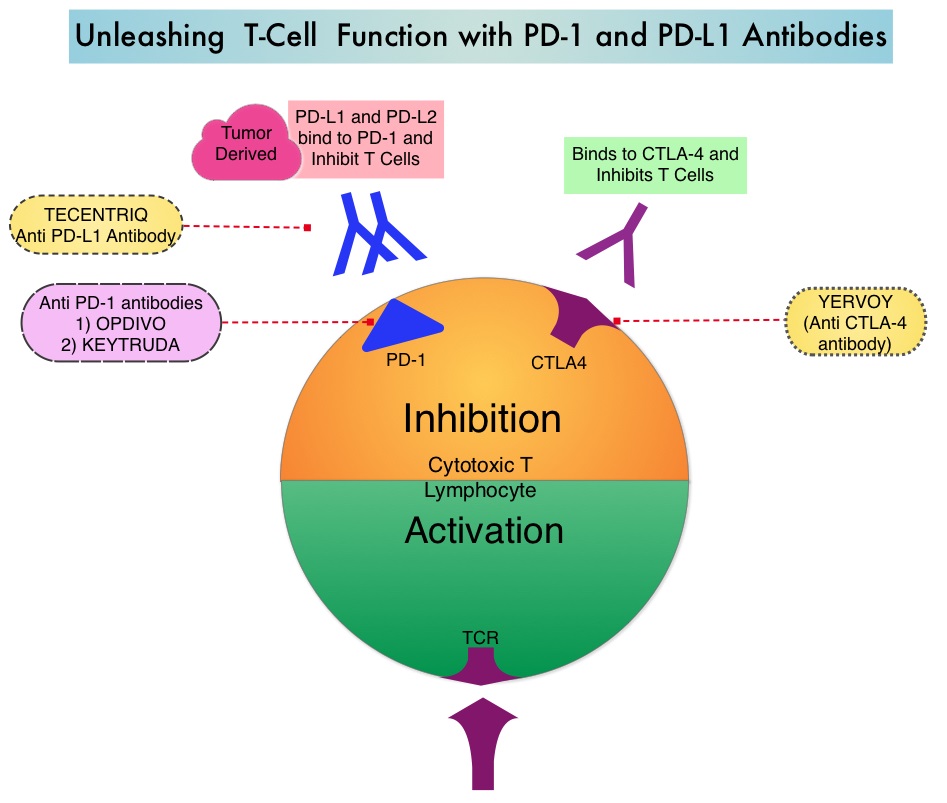 The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
KEYTRUDA® is a fully humanized, Immunoglobulin G4, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. The accelerated approval of KEYTRUDA® was based on a multicenter, nonrandomized, open-label, multi-cohort phase Ib study (KEYNOTE-012), which included 192 patients with recurrent or metastatic HNSCC. Approximately 33% of the patients were HPV positive and patients have a median of two prior lines of therapy. Almost all enrolled patients (95%) had prior radiation therapy. Median patient age was 60 years. Treatment consisted of KEYTRUDA® 10 mg/kg IV every 2 weeks or 200 mg IV every 3 weeks and continued until disease progression or unacceptable toxicities. Patients without disease progression were treated for up to 24 months. The primary end point was Objective Response Rate (ORR) and Duration of Response. Secondary endpoints included response by HPV status, Progression Free Survival (PFS), and safety. Efficacy was evaluated in 174 of the enrolled patients. The ORR was 16% with a Complete Response Rate of 5%. The median response duration had not been reached at the time of analysis. Among the responding patients, 82% had responses of 6 months or longer. The ORR and Duration of Response were similar irrespective of dosage regimen or HPV status. In a pooled analyses after long term follow up, responses were ongoing in 76% of the patients with a median follow up duration in responders of 12.5 months. Median Overall Survival was 8.5 months and 6 month PFS rate was 24.9%. The most common adverse reactions ((20% or greater) were fatigue, decreased appetite, and dyspnea and these were similar to those occurring in patients with Melanoma or Non Small Cell Lung Cancer, with the exception of an increased incidence of facial edema and new or worsening hypothyroidism.
It was concluded that KEYTRUDA® has significant antitumor activity in recurrent/metastatic Head and Neck Squamous Cell Carcinoma and PD-L1 testing is not needed prior to use of KEYTRUDA® for this indication. As a condition of the accelerated approval, a multicenter, randomized trial is to be conducted for continued approval, establishing the superiority of KEYTRUDA® over standard therapy. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): Pooled analyses after long-term follow-up in KEYNOTE-012. Mehra R, Seiwert TY, Mahipal A, et al. J Clin Oncol 34, 2016 (suppl; abstr 6012)

