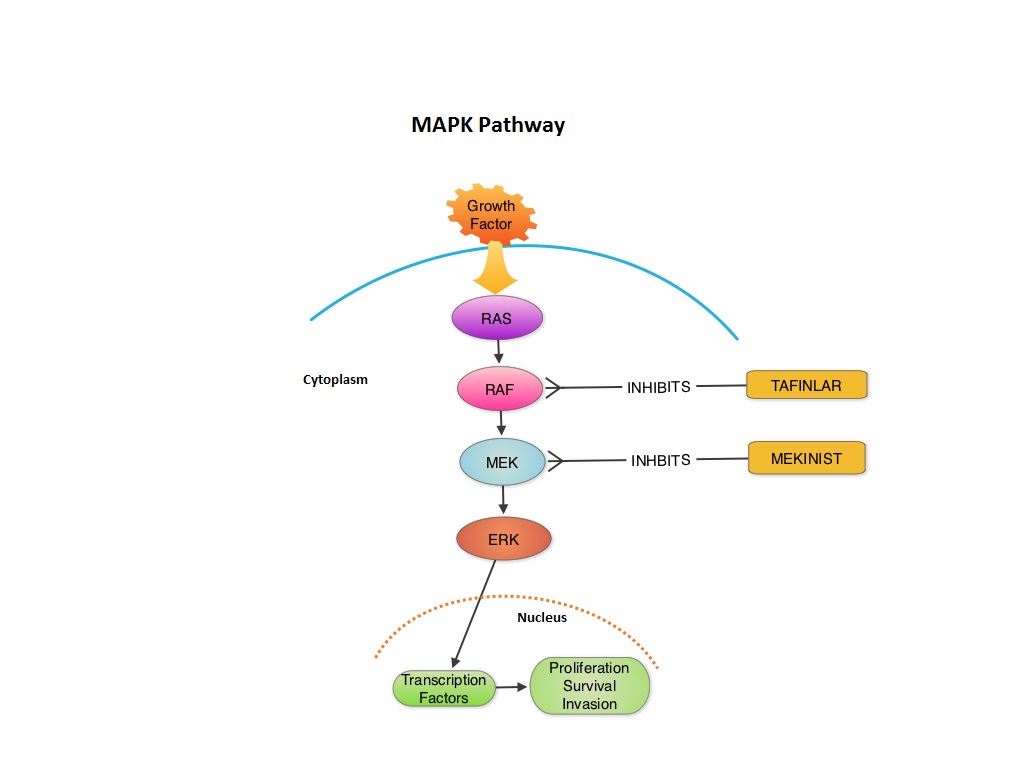
SUMMARY:The FDA granted accelerated approval in January 2014, for a combination of MEKINIST® (Trametinib) and TAFINLAR® (Dabrafenib), to treat patients with advanced melanoma, based on the understanding of the biological pathways of this malignancy. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6%-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E site and is detected in approximately 50% of melanomas. In the BREAK-3 randomized phase III trial, TAFINLAR®, a selective oral BRAF inhibitor demonstrated a statistically significant improvement in Progression Free Survival (PFS) and Response Rate (RR) compared to Dacarbazine (DTIC) in patients with advanced BRAF V600E mutated melanoma. Squamous cell carcinoma’s were seen in 6% of the patients. In the METRIC phase III study, MEKINIST®, a potent and selective inhibitor of MEK gene (which is downstream from RAF in the MAPK pathway) was compared with either Dacarbazine or TAXOL® (Paclitaxel) in advanced melanoma patients with BRAF V600E/K mutations. Patients in the MEKINIST® group had a significantly improved PFS, RR and Overall Survival. Based on the understanding of the biological pathways of the disease and different mechanisms of action (MOA) of these two agents, a phase I and II trial was conducted combining TAFINLAR® and MEKINIST®. In this study, 162 treatment naïve patients with unresectable or metastatic melanoma, with BRAF V600E or V600K mutations received either TAFINLAR® 150 mg plus MEKINIST® 1 or 2 mg or TAFINLAR® alone. Treatment was given until disease progression or side effects were intolerable. The primary end points were the incidence of Cutaneous Squamous-cell carcinoma, PFS and RR. Secondary end points included Overall Survival and pharmacokinetic activity. The combination treatment resulted in a lower incidence of Cutaneous Squamous-cell carcinoma (7% vs 19% in those receiving monotherapy, P=0.09), improved median PFS (9.4 months vs 5.8 months in the monotherapy group, HR=0.39, P<0.001) and superior complete or partial responses (76% compared with 54% with monotherapy (P=0.03). Pyrexia was however more common in the combination group than in the monotherapy group (71% vs. 26%). The authors concluded that by combining two agents with different MOA’s targeting two different tyrosine kinases in the RAS/RAF/MEK/ERK pathway, PFS was significantly improved. Further, development of resistance can be overcome in BRAF mutation positive melanoma patients and the incidence of secondary skin cancers found with BRAF inhibitor monotherapy can be reduced as well. Flaherty KT, Infante JR, Daud A, et al. N Engl J Med 2012;367:1694-1703