SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 135,430 new cases of ColoRectal Cancer will be diagnosed in the United States in 2017 and over 50,260 patients are expected to die of the disease. The lifetime risk of developing ColoRectal Cancer (CRC) is about 1 in 20 (5%). The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. The BRAF V600 mutations results in constitutive activation of the MAP kinase pathway. Inhibiting BRAF can transiently reduce MAP kinase signaling. However, this can result in feedback upregulation of EGFR signaling pathway, which can then reactivate the MAP kinase pathway. This aberrant signaling can be blocked by dual inhibition of both BRAF and EGFR.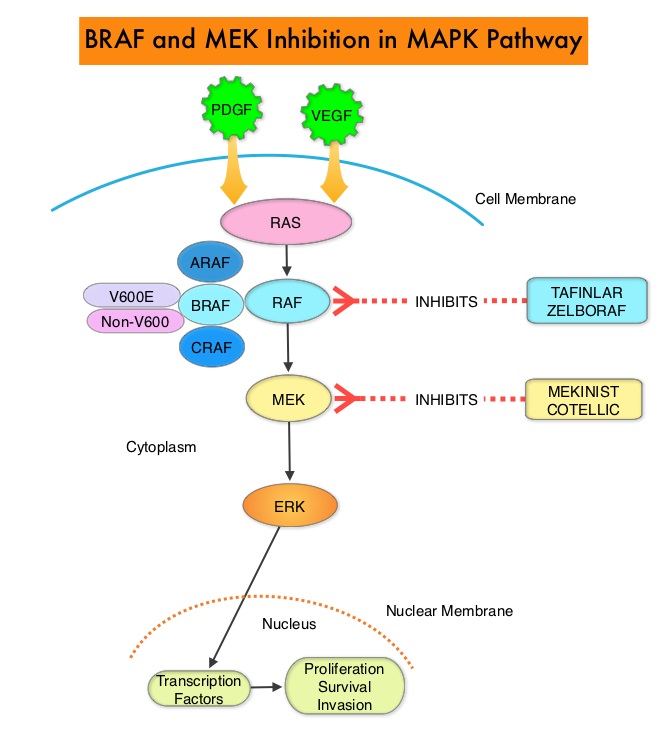
The initial evaluation of patients with metastatic ColoRectal Cancer (CRC) includes Molecular diagnostic testing including testing for extended RAS (RAt Sarcoma) and RAF (Rapid Accelerated Fibrosarcoma) mutations. Next-Generation Sequencing (NGS) allows expanded mutational testing for RAS which includes KRAS, NRAS and HRAS. RAS mutations are predictive of resistance to EGFR targeted therapy. NGS is able to detect approximately 20% of the patients who were originally classified as having KRAS Wild-Type (WT) metastatic CRC but were subsequently found to have KRAS or NRAS mutations, thus predicting resistance to EGFR targeted therapy.
Approximately 10% of CRCs detected by NGS harbor BRAF V600E mutation and the detection of BRAF V600E mutation is recognized as a marker of poor prognosis in patients with metastatic CRC. RAS and BRAF V600E mutations occur in a mutually exclusive fashion. Patients with this molecular subtype of CRC are older than age 60 years, more frequently female, have a right-sided tumor with high-grade histology, and often have MicroSatellite Instability (MSI-H). These patients often have peritoneal metastasis and despite chemotherapeutic intervention have a shortened overall survival. These tumors have limited response to EGFR targeted therapy and current guidelines recommend against the use of anti-EGFR antibodies in BRAF V600E-mutated mCRC.
The significance of non-V600 BRAF mutations detected by NGS however has remained unclear. The authors in this multicenter, retrospective cohort study, pooled NGS data from three large US reference laboratories and attempted to establish the clinical characteristics of patients with non-V600 BRAF mutations. Using NGS databases from the Mayo Clinic (MC), The University of Texas MD Anderson Cancer Center (MDACC), and Foundation Medicine (FM) from 2013-2016, patients with non-V600 BRAF mutations from these three institutions were identified and pooled for the primary analysis. Out of a total of 9,643 patients with metastatic CRC who underwent NGS testing, 208 patients with non-V600 BRAF mutations and 133 patients with V600E BRAF mutations, were identified. This study also included 249 patients with Wild-Type BRAF metastatic CRC, for comparative analysis , identified from the same NGS database, from the Mayo Clinic.
It was noted that the prevalence rate of any BRAF mutation was 10%, non-V600 BRAF mutations occurred in 2.2% of all patients tested and accounted for 22% of all BRAF mutations identified. Of particular clinical interest, compared with those with V600E BRAF mutations, patients harboring a non-V600 BRAF mutation were significantly younger, more frequently male, and presented with left-sided MicroSatellite-Stable (MSS) tumors. Additionally, non-V600 BRAF-mutated tumors were mostly low grade and did not often metastasize to the peritoneum. The median Overall Survival was significantly longer in patients with non-V600 BRAF-mutant metastatic CRC compared with those with both V600E BRAF-mutant and Wild-Type BRAF metastatic CRC (60.7 vs 11.4 vs 43.0 months, respectively; P<0.001) and in multivariable analysis, non-V600 BRAF mutation was independently associated with improved Overall Survival (HR=0.18; P<0.001).
This study concluded that Non-V600 BRAF mutations in metastatic ColoRectal Cancer (CRC), which accounted for 22% of all BRAF mutations identified by NGS, is a clinically distinct subtype of CRC, with an excellent prognosis and aggressive chemotherapeutic intervention could be avoided for this group of patients. Non-V600 BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. Jones JC, Renfro LA, Al-Shamsi HO, et al. J Clin Oncol 2017;35: 2624-2630

