The FDA on February 16, 2024, granted accelerated approval to AMTAGVI®, a tumor-derived autologous T cell immunotherapy, for adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 positive, a BRAF inhibitor with or without a MEK inhibitor. AMTAGVI® is a product of Iovance Biotherapeutics, Inc.
Tag: Malignant Melanoma of the Skin
FDA Approves Nivolumab for Adjuvant Treatment of Stage IIB/C Melanoma
SUMMARY: The FDA on October 13, 2023, approved Nivolumab (OPDIVO®) for the adjuvant treatment of completely resected Stage IIB/C melanoma in patients 12 years and older. The American Cancer Society’s estimates that for 2023, about 97,610 new cases of melanoma of the skin will be diagnosed in the United States and 7,990 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma.
Patients with resected Stage IIB/C disease comprise a significant group of patients at significant risk of recurrence. Patients with Stage IIB disease have primary tumors that are more than 2 mm and 4 mm or less in thickness with ulceration (T3b) or more than 4 mm in thickness without ulceration (T4a). Patients with Stage IIC disease have primary tumors more than 4 mm in thickness with ulceration (T4b). Although Stage II melanoma is less advanced than Stage III, the 5-year risk of recurrence in patients with stage IIB or Stage IIC disease without adjuvant therapy is approximately 35% and 50% respectively. The 5-year Melanoma-Specific Survival (MSS) rates for patients with Stage IIB/IIC disease are similar to those for Stage IIIA, Stage IIIB and Stage IIIC disease.
Immune Checkpoint Inhibitors are the standard of care adjuvant treatment for high-risk, resected, Stage III melanoma. In the KEYNOTE-054 trial, the 5-year Relapse Free Survival (RFS) with adjuvant Pembrolizumab was 55.4% versus 38.3% with placebo, in patients with completely resected, Stage IIIA (more than 1 mm lymph node metastasis), IIIB or IIIC Melanoma. In the CHECKMATE-238 trial, the 4-year RFS rate was of 51.7% for Nivolumab versus 41.2% for ipilimumab among patients with resected Stage IIIB/C and IV melanoma.
CHECKMATE-76K is an ongoing, randomized, double-blind, Phase III study conducted to evaluate the efficacy of Nivolumab versus placebo as adjuvant treatment for patients with resected Stage IIB/C melanoma. In this study, 790 eligible patients were randomized (2:1) to Nivolumab 480 mg (N=526) or placebo (N=264) by IV infusion every 4 weeks for up to 1 year or until disease recurrence or unacceptable toxicity. Patient characteristics at baseline were well balanced between treatment groups and 50.5% had nodular melanoma, 39% had Stage IIC disease and patients were stratified by tumor category. The Primary endpoint was investigator-assessed Recurrence-Free Survival (RFS). Secondary endpoints included Distant Metastasis-Free Survival (DMFS) and Safety.
At a minimum follow up of 7.8 months, CheckMate 76K met its primary endpoint and Nivolumab significantly improved RFS versus placebo. Nivolumab demonstrated a 58% reduction in the risk of recurrence or death versus placebo in patients with resected stage IIB/C melanoma (HR = 0.42; P < 0.0001). The 12-month RFS was 89.0% for nivolumab and 79.4% for placebo. The benefit with nivolumab over placebo was observed across all pre-specified subgroups, including all disease Stages and T-category subgroups. Adjuvant Nivolumab demonstrated significant benefit in those with stage IIC disease, head and neck primaries or nodular disease, who are all considered to be at a higher absolute recurrence risk. Additionally, there was a clinically meaningful improvement in the Distant Metastasis-Free Survival with Nivolumab versus placebo (HR = 0.47). Further, a lower proportion of patients treated with nivolumab had multiple lesions detected at first recurrence versus those treated with placebo (3.4% versus 9.1%). Adverse events were similar to that observed in patients with resected stage III or stage IV disease and similar to the established anti-PD-1 monotherapy profile.
It was concluded that adjuvant Nivolumab significantly improved Relapse Free Survival as well as Distant Metastasis-Free Survival in patients with resected Stage IIB/C melanoma and this clinical benefit was observed across disease subgroups, including all T categories.
Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized, phase 3 CheckMate 76K trial. Kirkwood, J.M., Del Vecchio, M., Weber, J. et al. Nat Med (2023). https://doi.org/10.1038/s41591-023-02583-2
Personalized mRNA Cancer Vaccine in Combination with KEYTRUDA® Improves Relapse Free Survival in Resected High Risk Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2023, about 97,610 new cases of melanoma of the skin will be diagnosed in the United States and 7,990 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma.
Immune Checkpoint Inhibitors are the standard of care adjuvant treatment for high-risk resected melanoma. In the KEYNOTE-054 trial, the 5-year Relapse Free Survival (RFS) with adjuvant Pembrolizumab was 55.4% versus 38.3% with placebo. In the CHECKMATE-238 trial, the 4-year RFS rate was of 51.7% for Nivolumab versus 41.2% for ipilimumab. Given the high relapse rates with the present adjuvant melanoma therapies, there is an unmet clinical need.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response.
mRNA-4157 (V940) is a novel messenger RiboNucleic Acid (mRNA)-based individualized neoantigen therapy consisting of a single synthetic mRNA coding for up to 34 neoantigens, that is designed and produced based on the unique mutational signature of the DNA sequence of the patients tumor. Individualized neoantigen therapies are designed to prime the immune system so that a patient can generate a tailored antitumor response specific to their tumor mutation signature. mRNA-4157 (V940) was designed to stimulate an immune response by generating specific T cell responses based on the unique mutational signature of a patient’s tumor. Early clinical studies demonstrated that combining mRNA-4157 (V940) with Pembrolizumab may potentially provide an additive benefit and enhance T cell-mediated destruction of tumor cells.
KEYNOTE-942 is a randomized Phase IIb trial, which assessed the efficacy of mRNA-4157/V940 in prolonging RFS in patients with resected, Stages IIIB/IIIC/IIID and IV melanoma, when given in combination with Pembrolizumab, the standard of care adjuvant therapy in this patient population. This study included 157 patients who were randomly assigned (2:1) to receive mRNA-4157/V940 in combination with Pembrolizumab (107 patients) or Pembrolizumab alone (50 patients). The vaccine was administered every three weeks for a total of nine doses, and Pembrolizumab was given at 200 mg IV every three weeks for up to 18 cycles (approximately one year). All patients had tumor sample (Formalin Fixed Paraffin Embedded-FFPE) available for Next Generation Sequencing and patients were stratified by disease stage. mRNA-4157/V940 was successfully prepared for more than 99% of patients in the combination arm. The median patient age was 62 years and 84% of patient had Stage IIIC disease. Approximately 64% of patients were PD-L1 positive and 74% had high Tumor Mutational Burden-TMB (10 or more mutations/Mb) in the combination treatment group, and 54% were PD-L1 positive and 60% had high TMB in the single agent Pembrolizumab group, respectively. The Primary endpoint was Relapse Free Survival (RFS), defined as the time from first dose of Pembrolizumab until the date of first recurrence (local, regional, or distant metastasis), a new primary melanoma, or death from any cause. Secondary endpoints included distant Metastasis-Free Survival and Safety. Exploratory endpoints included distribution of TMB expression in baseline tumor samples across study arms and their association with the primary RFS endpoint. The median follow up was 23 months for the mRNA-4157/V940 plus Pembrolizumab group and 24 months for Pembrolizumab alone group.
The Relapse Free Survival at 18 months was 78.6% for the immunotherapy combination versus 62.2% for Pembrolizumab alone (HR=0.56; P=0.0266), and this equated to a 44% reduction in the risk of recurrence or death with 2 years of follow up. mRNA-4157/V940 and Pembrolizumab combination treatment demonstrated an improvement in RFS, irrespective of PD-L1 status and TMB status. The immunotherapy combination was well tolerated without increased Grade 3-4 immune mediated or serious toxicities. The most common adverse events of any grade attributed to the combination immunotherapy were fatigue, injection site pain and chills.
The researchers concluded that this is the first randomized trial to demonstrate Relapse Free Survival improvement with an individualized neoantigen approach, compared to standard of care treatment with Pembrolizumab, among patients with high-risk resected melanoma.
A personalized cancer vaccine, mRNA-4157, combined with pembrolizumab versus pembrolizumab in patients with resected high-risk melanoma: Efficacy and safety results from the randomized, open-label Phase 2 mRNA-4157-P201/Keynote-942 trial. Khattak A, Carlino M, Meniawy T, et al. Presented at: 2023 AACR Annual Meeting; April 14-19, 2023; Orlando, FL. Abstract CT001.
Tumor-Infiltrating Lymphocyte Therapy in Advanced Refractory Melanoma
SUMMARY: The American Cancer Society estimates that in 2022, about 99,780 new cases of melanoma of the skin were diagnosed in the United States and 7,650 people died of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate (ORR) and prolongation of survival across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. YERVOY® (Ipilimumab) is a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4, and was the first systemic therapy in randomized Phase III trials, to show prolonged Overall Survival (OS) in patients with advanced melanoma. The two PD-1 inhibitors of interest are OPDIVO® (Nivolumab) and KEYTRUDA® (Pembrolizumab), which are fully human, Immunoglobulin G4, anti-PD-1 targeted monoclonal antibodies that bind to the PD-1 receptor, and block its interaction with ligands PD-L1 and PD-L2, following which the tumor-specific effector T cells are unleashed. They are thus able to undo PD-1 pathway-mediated inhibition of the immune response. When compared with YERVOY® in patients with advanced melanoma, PD-1 inhibitors, both OPDIVO® and KEYTRUDA® have demonstrated superior Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR), with a better safety profile. They are therefore frequently used first-line treatment in patients with metastatic melanoma.
Over 50% of untreated patients receiving a combination of PD-1 and CTLA-4 inhibitors are alive after five years. However, combination immunotherapy with YERVOY® and OPDIVO® is associated with a high incidence of severe adverse events and is currently recommended primarily for a subgroup of patients with poor prognostic factors such as a high serum LDH levels or liver or brain metastases. Approximately 50% of melanomas harbor BRAF V600E mutation and are often treated with a combination of BRAF and MEK inhibitors. This combination is associated with a high response, but resistance develops in most patients over time. YERVOY® is presently often used as second line therapy, but only 15-30% of patients benefit from this intervention. There is an unmet need for this group of patients.
Adoptive immunotherapy, also known as cellular immunotherapy, is a form of treatment in which naturally occurring or gene-engineered T cells with antitumor activity are transferred to a tumor-bearing host to eliminate cancer. These killer T cells bind to antigens on the surface of cancer cells and destroy them. Cellular immunotherapies include Tumor-Infiltrating Lymphocyte (TIL) Therapy, Engineered T Cell Receptor (TCR) Therapy, Chimeric Antigen Receptor (CAR) T Cell Therapy and Natural Killer (NK) Cell Therapy.
Adoptive immunotherapy with Tumor-Infiltrating Lymphocytes (TILs) is a personalized autologous therapy in which lymphocytes which have infiltrated the tumor are expanded in vitro and administered intravenously following nonmyeloablative, lymphodepleting chemotherapy, and supported by the IV administration of Interleukin-2 (IL-2) to enhance the in vivo expansion of the cells and augment antitumor responses. In contrast to Lymphokine-Activated Killer cells (LAK), human TILs demonstrate cytolytic specificity against only the tumor from which they were derived or against closely related tumors, and in preclinical models have proved to be 50 to 100 times more potent than LAK cells. Evidence of clinical activity of TIL therapy in patients with advanced melanoma was initially reported by Rosenberg and colleagues in the 1990s and subsequent Phase 1-2 trials showed responses in 30-70% of patients, with responses noted even among those who had disease progression while receiving anti-PD1 treatment. Nonetheless, there has been no direct comparison of TILs with standard treatment.
This multicenter, open-label, Phase III, randomized trial was conducted to compared TILs with Yervoy® as first or second-line treatment in patients with advanced melanoma. In this study, a total of 168 patients with unresectable Stage IIIC or IV melanoma were randomly assigned in a 1:1 ratio to receive either TILs (N=84) or YERVOY® (N=84). Patients assigned to receive TILs underwent metastasectomy for the retrieval and expansion of TILs, followed by inpatient administration of nonmyeloablative, lymphodepleting chemotherapy, which consisted of Cyclophosphamide 60 mg/kg IV QD for 2 days and Fludarabine 25 mg/m2 IV QD for 5 days, single adoptive transfer of 5×109 to 2×1011 TILs intravenously, and subsequent high-dose IL-2, 600,000 IU/kg IV every 8 hours, for a maximum of 15 doses. Patients in the YERVOY® group received 3 mg/kg IV every 3 weeks, for a maximum of 4 doses. Administration of YERVOY® could be delayed or discontinued if adverse events occurred, and no dose reductions were allowed. Both treatment groups were well balanced and 86% of patients were refractory to PD-1 inhibitor therapy, mostly adjuvant or first line therapy. The median patient age was 59 years and patients were stratified according to BRAF V600-mutation status, line of treatment, and treatment center. The Primary end point was Progression Free Survival (PFS). Secondary end points included Objective Response Rate (ORR), Complete Response (CR), Overall Survival (OS), Health-Related Quality of Life and Safety.The median follow-up was 33.0 months.
The median PFS was 7.2 months in the TIL group and 3.1 months in the YERVOY® group (HR=0.50;P<0.001).The Objective Response Rate was 49% in the TIL group and 21% in the YERVOY® group, with a Complete Response rate of 20% in the TIL group and 7% in the YERVOY® group, with durable Complete Responses in both treatment groups. The median Overall Survival was 25.8 months in the TIL group and 18.9 months in the YERVOY® group(HR=0.83). The 2-year OS was 54.3% in the TIL group and 44.1% in the YERVOY® group. Treatment-related adverse events of Grade 3 or higher occurred in all patients in the TIL group and in 57% of those in the YERVOY® group, and these events were mainly chemotherapy-related myelosuppression. Treatment-related serious adverse events occurred in 15% of the patients in the TIL group and 27% of those in the YERVOY® group.
It was concluded that in patients with advanced melanoma including those patients refractory to PD-1 inhibitor therapy, treatment with TILs was associated with significantly longer Progression Free Survival than treatment with YERVOY®.
Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. Rohaan MW, Borch TH, Van den Berg JH, et al. N Engl J Med 2022; 387:2113-2125
TAFINLAR® and MEKINIST® versus OPDIVO® plus YERVOY® for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial
SUMMARY: The American Cancer Society estimates that for 2022, about 99,780 new cases of melanoma of the skin will be diagnosed in the United States and 7,650 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been detected in 6-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas, and results in constitutive activation of the MAPK pathway.
Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate (ORR) and prolongation of survival across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. Over 50% of patients treated with a combination of PD-1 and CTLA-4 inhibitors are alive after five years.
TAFINLAR® (Dabrafenib), is a selective oral BRAF inhibitor and MEKINIST® (Trametinib) is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway. TAFINLAR® plus MEKINIST® led to long-term survival benefit in approximately one third of the patients who had unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, from two randomized Phase III COMBI-d and COMBI-v trials.
A combination of OPDIVO® (Nivolumab) plus YERVOY® (Ipilimumab) showed durable improved outcomes among patients with unresectable or metastatic melanoma and approximately 50% of patients were alive at 6.5 years (J Clin Oncol 39, 2021. suppl 15; abstr 9506). The FDA granted approval for this combination in 2015 for the treatment of patients with metastatic melanoma, regardless of tumor BRAF mutation status.
It has been noted that BRAF/MEK inhibitor therapy tends to produce high tumor response rates and prolonged median Progression Free Survival (PFS), whereas OPDIVO® /YERVOY® tends to have its major impact on Duration of Response. However, the optimal treatment sequence for patients with treatment-naive BRAFV600-mutant metastatic melanoma, between combination OPDIVO®/YERVOY® checkpoint inhibitor immunotherapy and combination TAFINLAR® plus MEKINIST® molecularly targeted therapy, has remained unclear. Recently published tumor biology studies have suggested that resistance to BRAF/MEK-inhibitor therapy results in an immunosuppressive tumor microenvironment that is void of functional CD103+ dendritic cells, preventing effective antigen presentation to the immune system, and that immunotherapy may enhance BRAF-mutated melanoma responsiveness to targeted therapy.
DREAMseq (EA6134) is a two-arm, two-step, open-label, randomized Phase III trial, which investigated the anti PD-1/CLTA-4 immunotherapy combination of OPDIVO® plus YERVOY® followed by the anti-BRAF/MEK targeted therapy combination of TAFINLAR® plus MEKINIST®, versus the reverse sequence, in patients with advanced BRAF V600-mutant melanoma. This study was conducted to determine which treatment sequence produced the best efficacy.
In this study, 265 patients with treatment-naive BRAF V600-mutant metastatic melanoma were randomly assigned to receive either combination OPDIVO® plus YERVOY® (arm A=133) or TAFINLAR® plus MEKINIST® (arm B=132) in step 1, and at disease progression were enrolled in step 2 to receive the alternate therapy, TAFINLAR® plus MEKINIST® (arm C=27) or OPDIVO® plus YERVOY® (arm D=46). The two initial treatment arms were balanced and more patients on arm B had BRAF V600K-mutant tumors than those on arm A (25.2% versus 12.1%). The median patient age was 61 years and eligible patients had histologically confirmed, BRAF V600-mutant unresectable Stage III or IV melanoma with measurable disease. The Primary end point was 2-year Overall Survival (OS). Secondary end points included 3-year OS, Objective Response Rate (ORR), Duration of Response, Progression Free Survival (PFS), crossover feasibility, and Safety.
The study was stopped early by the Independent Data Safety Monitoring Committee because statistical significance was achieved for the Primary endpoint. The 2-year OS for those starting on arm A was 71.8% and arm B was 51.5% (P=0.01). Step 1 Progression Free Survival favored arm A (P=0.054). The Objective Response Rates were arm A: 46%, arm B: 43%, arm C: 47.8%, and arm D: 29.6%. The median Duration of Response was not reached for arm A, and 12.7 months for arm B (P<0.001). Crossover occurred in 52% of patients following documented disease progression. Grade 3 or more toxicities occurred with similar frequency between treatment groups and adverse events related to regimens were as expected.
It was concluded from this study that for patients with advanced BRAF V600-mutant metastatic melanoma, the treatment sequence beginning with the immune checkpoint inhibitor combination of OPDIVO® plus YERVOY® resulted in superior Overall Survival and longer Duration of Response, compared with the treatment sequence beginning with TAFINLAR® plus MEKINIST®, and should therefore be the preferred treatment sequence for most of these patients.
Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial—ECOG-ACRIN EA6134. Atkins MB, Lee SJ, Chmielowski B, et al. J Clin Oncol. Published online September 27, 2022. doi:10.1200/JCO.22.01763
Association of Gut Microbiome with Immune Checkpoint Inhibitor Response in Advanced Melanoma
SUMMARY: The American Cancer Society estimates that in 2022, there will be an estimated 1.92 million new cancer cases diagnosed and 609,360 cancer deaths in the United States. Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate and prolongation of survival across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. Over 50% of patients treated with a combination of PD-1 and CTLA-4 inhibitors are alive after five years. Nonetheless, less than 50% of the patients respond to single-agent ICI and a higher response to targeting both PD-1 and CTLA-4 is associated with significant immune-related Adverse Events.
Biomarkers predicting responses to ICIs include Tumor Mutational Burden (TMB), Mismatch Repair (MMR) status, and Programmed cell Death Ligand 1 (PD-L1) expression. Other biomarkers such as Tumor Infiltrating Lymphocytes (TILs), TIL- Interferon-gamma, Neutrophil-to-ratio, and peripheral cytokines, have also been proposed as predictors of response. It has been postulated that concomitant medications during therapy with ICIs such as baseline steroid use as well as treatment with antibiotics may negate or lessen the efficacy of ICIs.
Preclinical studies have suggested that immune-based therapies for cancer may have a very complex interplay with the host’s microbiome and there may be a relationship between gut bacteria and immune response to cancer. The gut microbiome is unique in each individual, including identical twins. The crosstalk between microbiota in the gut and the immune system allows for the tolerance of commensal bacteria (normal microflora) and oral food antigens and at the same time enables the immune system to recognize and attack opportunistic bacteria. Immune Checkpoint Inhibitors strongly rely on the influence of the host’s microbiome, and the gut microbial diversity enhances mucosal immunity, dendritic cell function, and antigen presentation. Broad-spectrum antibiotics can potentially alter the bacterial composition and diversity of our gut microbiota, by killing the good bacteria. It has been postulated that this may negate the benefits of immunotherapy and influence treatment outcomes. It should be noted however that the relationship between gut bacteria and immune response is influenced by several factors and may be partially cancer type specific and it is unlikely that the same microbiome features can reflect the uniqueness of the genetic and immune characteristics of each tumor.
Even though the composition of the gut microbiome has been associated with clinical responses to immune checkpoint inhibitor (ICI) treatment, there is a lack of consistency of results between the published studies, and there is limited consensus on the specific microbiome characteristics linked to the clinical benefits of ICIs. The Predicting Response to Immunotherapy for Melanona with Gut Microbiome and Metabolomics (PRIMM) studies are two separate prospective observational cohort studies that has been recruiting patients in the UK (PRIMM-UK) and the Netherlands (PRIMM-NL) since 2018. These cohorts of previously ICI-naive patients with advanced melanoma have provided extensive biosamples, including stool, serum and peripheral blood mononuclear cells, before and during ICI treatment, with detailed clinical and dietary data collected at regular intervals longitudinally.
The authors therefore performed a meta-analysis on existing publicly available datasets to produce the largest study to date. In order to study the role of the gut microbiome in ICI response, the researchers recruited ICI-naive patients with advanced cutaneous melanoma from the PRIMM cohorts, as well as three additional cohorts of ICI-naive patients with advanced cutaneous melanoma, originating from Barcelona, Leeds and Manchester (N = 165), and performed shotgun metagenomic sequencing on a total of 165 stool microbiome samples collected before initiating ICI treatment. Shotgun sequencing is a laboratory technique for determining the DNA sequence of an organism’s genome. This dataset was integrated with 147 metagenomic samples from smaller publicly available datasets. This methodology provided the largest assessment of the potential of the gut microbiome as a biomarker of response to ICI, in addition to allowing for investigation of specific microbial species or functions associated with response. Patient demographics including age, gender, BMI, previous non-immunotherapy treatments, previous drug therapies such as antibiotics, Proton Pump Inhibitors (PPIs) and steroids, as well as dietary patterns, were collected in these cohorts for the majority of patients, and were considered in the multivariate analysis.
The researchers used machine learning analysis to understand the association between gut microbiome and response to ICIs. This analysis confirmed the link between the microbiome and Overall Response Rates (ORRs), as well as Progression Free Survival (PFS) with ICIs. This analysis also revealed limited reproducibility of microbiome-based signatures across cohorts. A panel of species, including Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansiamuciniphila were associated with responders, but no single species could be regarded as a fully reliable biomarker across studies. Based on these findings from this large set of real-world cohorts, the authors noted that the relationship between human gut microbiome and response to ICIs is more complex than previously understood, and extends beyond the presence or absence of different microbial species in responders and nonresponders.
It was concluded that future studies should include large samples and take into account the complex interplay of clinical factors with the gut microbiome over the treatment course. Until then, the authors recommend high-quality, diverse, whole-foods diet to optimize gut health, rather than consumption of commercial probiotics.
Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Lee KA, Thomas AM, Bolte LA, et al. Nat Med. 2022;28:535-544.
Opdualag™ (nivolumab and relatlimab-rmbw): A New Dual I-O Option in the 1L Treatment of Metastatic Melanoma
Written By: Leonel Fernando Hernandez Aya, MD. Division of Medical Oncology, Department of Medicine, University of Miami Miller School of Medicine, Sylvester Comprehensive Cancer Center
Content Sponsored by: Bristol Myers Squibb
Dr Hernandez Aya is a paid consultant for BMS and was compensated for his contribution in drafting this content.
See additional definitions of abbreviations used throughout the article at the bottom of this page.
Overview of Metastatic Melanoma
Since the approval of anti–CTLA-4 in 2011 for metastatic melanoma, immuno-oncology(I-O) has transformed treatment outcomes.1 There are now several approved I-O options, and of those approved for the treatment of metastatic melanoma, dual immunotherapy in particular has had long-term success.2 The first dual immunotherapy, approved in 2015, consisted of PD-1 and CTLA-4 checkpoint inhibitors for the 1L treatment of unresectable or metastatic melanoma, regardless of BRAF mutation status.1,3,4 This anti–PD-1 and anti–CTLA-4 combination showed benefit in overall survival (OS) compared with anti–CTLA-4 alone.5 In general, the safety profile was consistent with previous experience with anti–PD-1 or anti–CTLA-4 alone.4 Until March 2022, this dual anti–PD-1 and anti–CTLA-4 immunotherapy was the only option indicated for the 1L treatment of unresectable or metastatic melanoma.3,6 Opdualag, the second approved dual immunotherapy, has provided an additional treatment option for nivolumab-monotherapy–appropriate patients with unresectable or metastatic melanoma.6-8
Opdualag
Opdualag is a dual immunotherapy option combining an anti–PD-1, nivolumab, with the first-in-class anti–LAG-3, relatlimab, in a fixed-dose formulation.7,8 PD-1 and LAG-3 are two distinct inhibitory immune checkpoints.7 Combined PD-1 and LAG-3 inhibition results in increased T-cell activation compared to the activity of either antibody alone. This initiates an improved anti-tumor immune response.8
Opdualag is indicated for the treatment of adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma.8 The approval is based on RELATIVITY-047, a phase 3, randomized, double-blind, global study of Opdualag versus nivolumab monotherapy.7 Patients were stratified by AJCC v8 M stage, BRAF, PD-L1, and LAG-3 status.7 Key exclusion criteria include patients with active or untreated brain or leptomeningeal metastases, uveal melanoma, active autoimmune disease, or medical conditions requiring systemic treatment with moderate- or high-dose corticosteroids or immunosuppressive medications.8
RELATIVITY-047 enrolled 714 patients who were randomized 1:1 to receive Opdualag (480 mg nivolumab/160 mg relatlimab as a fixed-dose combination[FDC]) every 4 weeks (n=355) or nivolumab 480 mg every 4 weeks (n=359).8 The primary endpoint was progression-free survival(PFS), and secondary endpoints were OS and overall response rate(ORR). PFS was determined by BICR using RECIST v1.1. Baseline characteristics were balanced across both treatment arms.7
Study design8
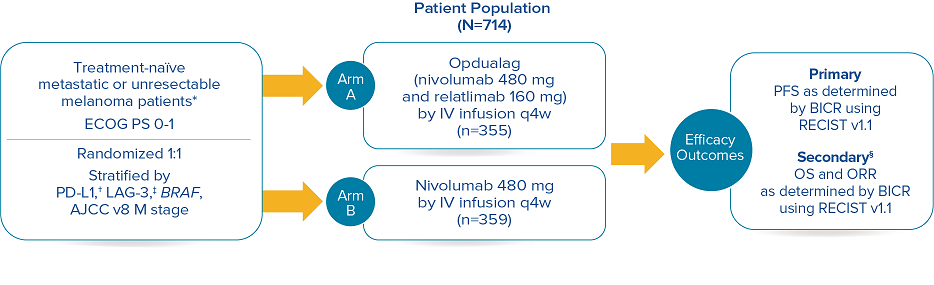
Median duration of treatment for Opdualag at the 19.3-month median follow-up was 8.3 months.7,9 Treat until disease progression or unacceptable toxicity.8
*Patients were allowed to have received prior adjuvant and neoadjuvant melanoma therapy. Anti–PD-1, anti–CTLA-4, or BRAF-MEK therapy was allowed as long as there was at least 6 months between the last dose of therapy and date of recurrence; interferon therapy was allowed as long as the last dose was at least 6 weeks prior to randomization.8† PD-L1 expression (≥1% vs <1%) using PD-L1 IHC 28-8 pharmDx test.8‡ LAG-3 expression (≥1% vs <1%) using a clinical trial assay.8§ The final analysis of OS was not statistically significant.8
Opdualag is associated with the following Warnings and Precautions: severe and fatal immune-mediated adverse reactions (IMARs) including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis with renal dysfunction, dermatologic adverse reactions, myocarditis, and other immune-mediated adverse reactions; infusion-related reactions; complications of allogeneic hematopoietic stem cell transplantation (HSCT); and embryo-fetal toxicity.
Opdualag demonstrated superior PFS compared to nivolumab at the primary analysis(median of 13.2 months) with curve separation as early as 3 months and sustained over time.7,8 Median PFS (mPFS)was 10.1 months with Opdualag versus 4.6 months with nivolumab (HR=0.75; 95% CI: 0.62–0.92; P=0.0055).8 Similarly, patients who received Opdualag had longer PFS regardless of key prognostic indicators, such as the AJCC metastasis stage of the tumor, LDH level, and tumor burden.7
At the follow-up analysis (median of 19.3 months), mPFS was 10.22 months with Opdualag and 4.63 months with nivolumab (HR=0.78; 95% CI: 0.64-0.94).10 OS and ORR were also evaluated.8 The final analysis for the secondary endpoint of OS was not statistically significant (threshold for significance was P<0.04302), and median OS (mOS)was not reached with Opdualag compared with nivolumab, which resulted in a mOS of 34.1 months (HR=0.80; 95% CI: 0.64–1.01; P=0.0593). Additionally, the ORR was higher with Opdualag (43%) versus nivolumab (33%), with the median DOR not yet reached for both treatment arms.8,10 ORR was not formally tested based on the testing hierarchy.8
Progression-free survival at the 19.3-month median follow-up10*†‡
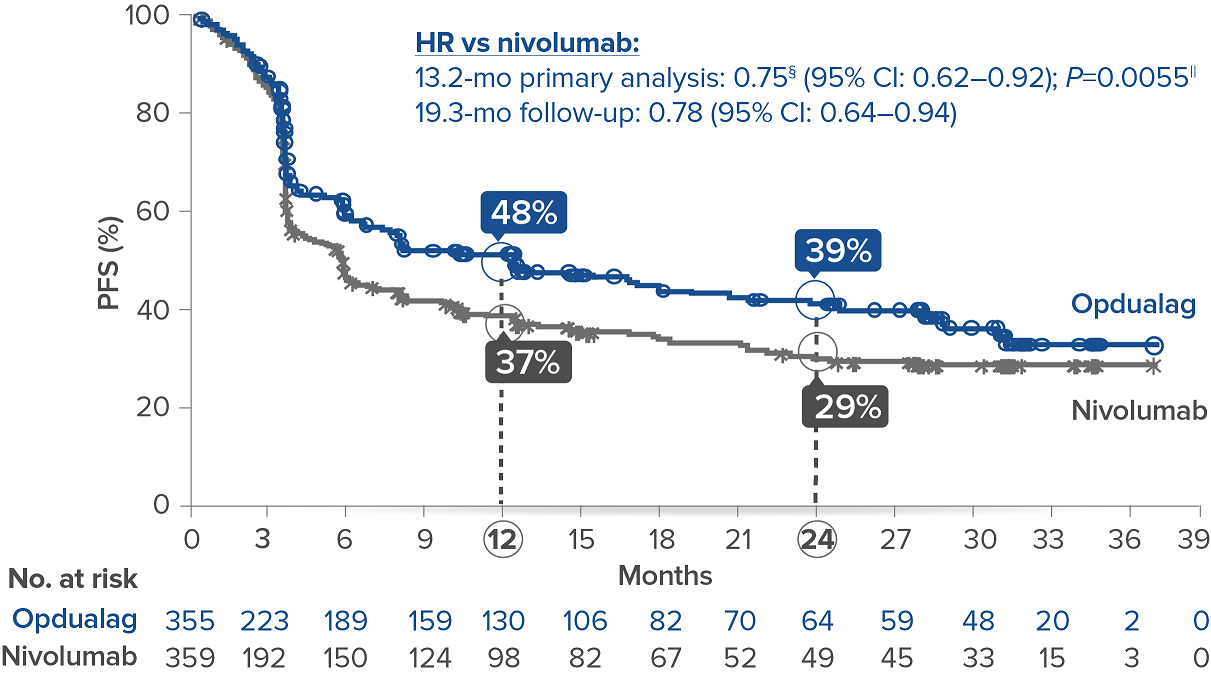
Symbols represent censored observations.
*Assessed by BICR.8† Final PFS analysis.8‡ Kaplan-Meier estimate.8§ Based on stratified Cox proportional hazard model.8II Based on stratified log-rank test.8
Overall survival10*
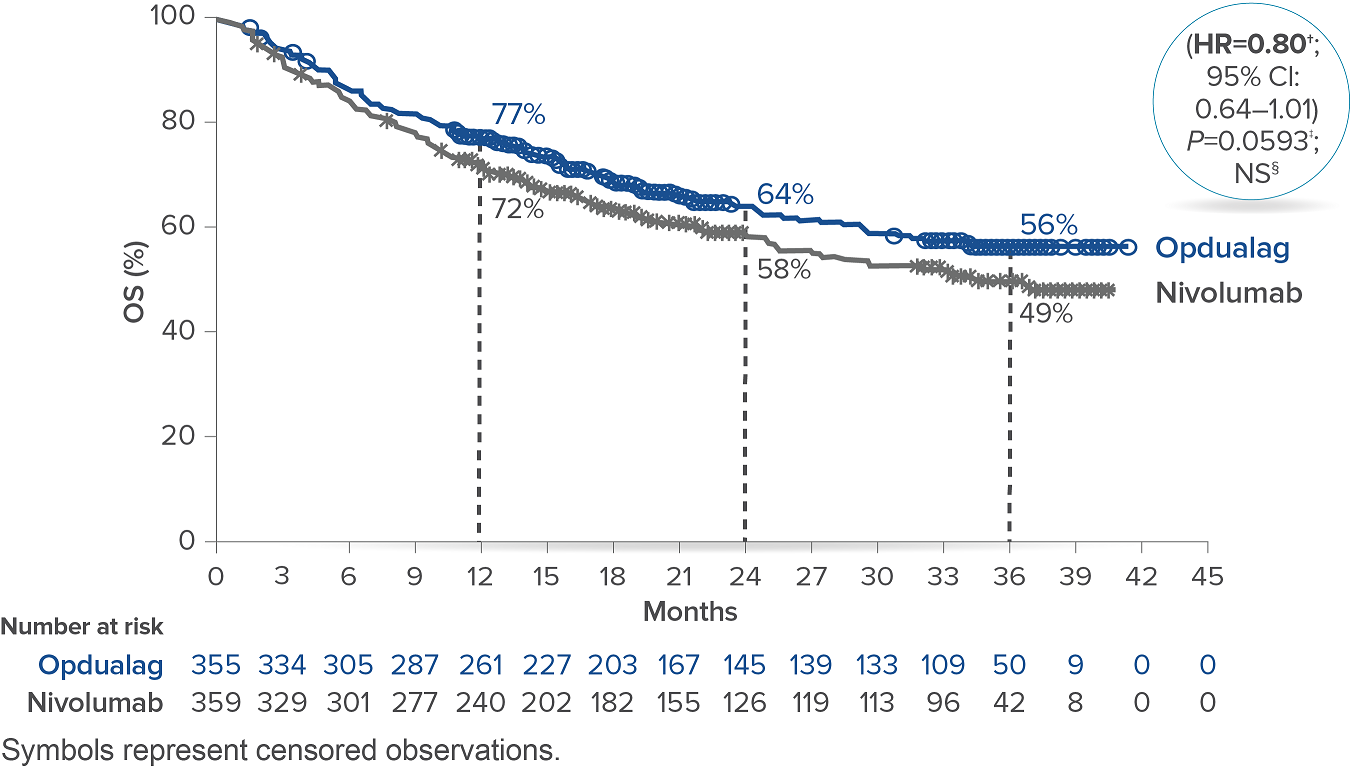
*At the time of the final OS analysis, which was event-driven and occurred after the final PFS analysis.8† Based on stratified Cox proportional hazard model.8‡ Based on stratified log-rank test.8§ Not significant at alpha level 0.04302.8
In RELATIVITY-047, Opdualag had no additional safety events and similar most common Grade 3/4 AEs versus nivolumab monotherapy.7,8 Adverse reactions occurring in ≥15% of patients receiving Opdualag were musculoskeletal pain (45%), fatigue (39%), rash (28%), pruritus (25%), diarrhea (24%), nausea (17%), headache (18%), hypothyroidism (17%), decreased appetite (15%), and cough (15%).8
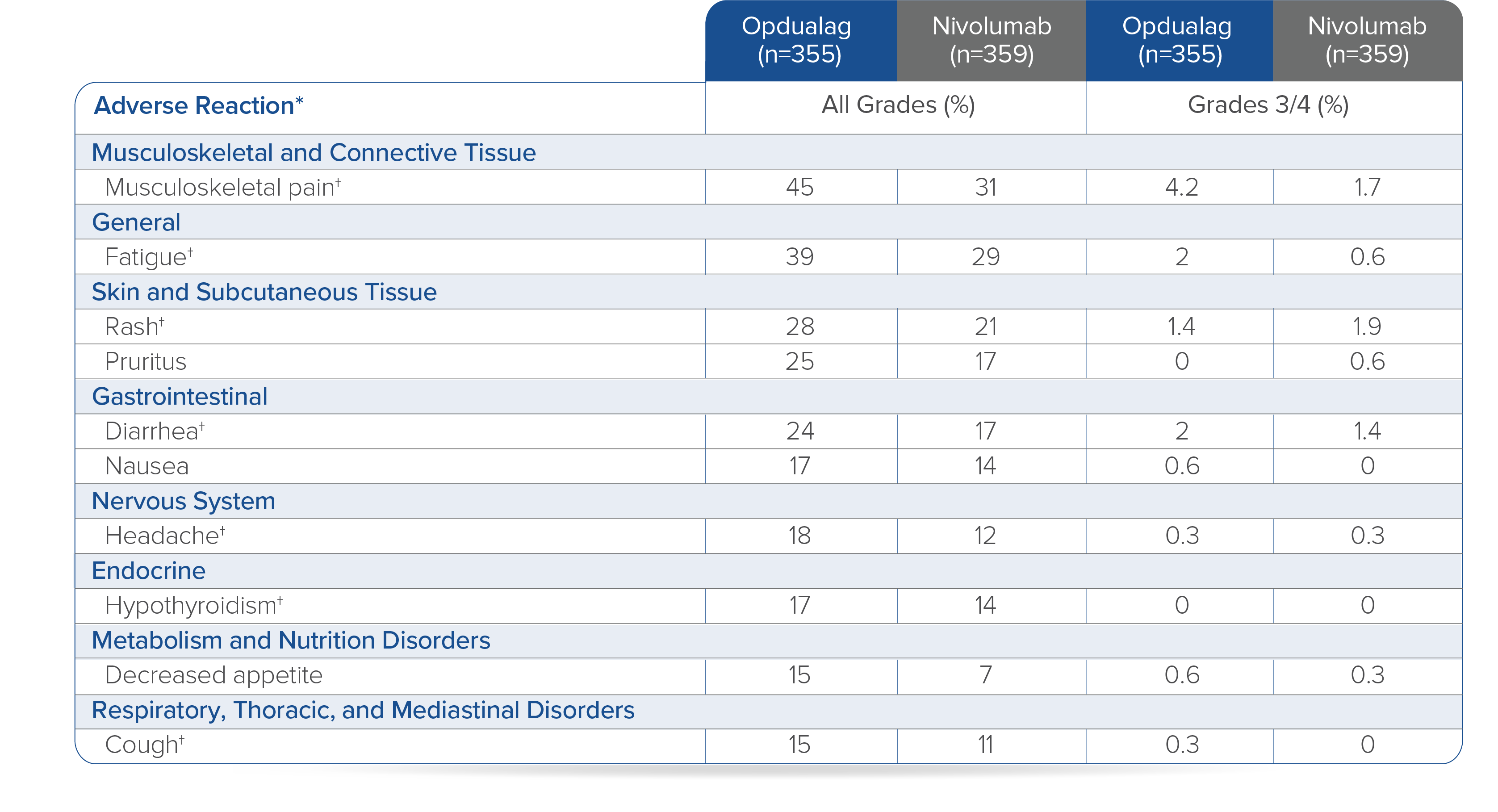
Toxicity was graded per NCI CTCAE v5.
*Clinically relevant adverse reactions in <15% of patients who received Opdualag included vitiligo, adrenal insufficiency, myocarditis, and hepatitis.8† Includes multiple terms.8
Opdualag is a FDC administered as a 30-minute intravenous infusion every 4 weeks.8 A FDC is the co-formulation of 2 active ingredients in a single vial administered as a single infusion, which may help reduce preparation and infusion times and could help minimize potential risk of administration errors.7,8,11 Opdualag can cause severe infusion-related reactions. Discontinue Opdualag in patients with severe or life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild to moderate infusion-related reactions. In patients who received Opdualag as a 60-minute intravenous infusion, infusion-related reactions occurred in 7% (23/355) of patients.8
Summary/conclusions
Dual immunotherapy has changed the metastatic melanoma treatment landscape.2 Currently there are 2 dual immunotherapy options available for 1L treatment of adult patients with unresectable or metastatic melanoma.3,8 As the newest dual immunotherapy, Opdualag more than doubled mPFS with a similar safety profile compared with nivolumab.8 Opdualag can be used for the treatment of all nivolumab monotherapy-appropriate patients, providing the opportunity for more patients with unresectable or metastatic melanoma to receive a dual immunotherapy.8 From my clinical experience, “it is great to have another treatment option for patients with metastatic melanoma.”
Indication for Opdualag
Opdualag is indicated for the treatment of adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma.
Important Safety Information for Opdualag
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions (IMARs) listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
IMARs which may be severe or fatal, can occur in any organ system or tissue. IMARs can occur at any time after starting treatment with a LAG-3 and PD-1/PD-L1 blocking antibodies. While IMARs usually manifest during treatment, they can also occur after discontinuation of Opdualag. Early identification and management of IMARs are essential to ensure safe use. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying IMARs. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected IMARs, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if Opdualag requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose IMARs are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
Opdualag can cause immune-mediated pneumonitis, which may be fatal. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Immune-mediated pneumonitis occurred in 3.7% (13/355) of patients receiving Opdualag, including Grade 3 (0.6%), and Grade 2 (2.3%) adverse reactions. Pneumonitis led to permanent discontinuation of Opdualag in 0.8% and withholding of Opdualag in 1.4% of patients.
Immune-Mediated Colitis
Opdualag can cause immune-mediated colitis, defined as requiring use of corticosteroids and no clear alternate etiology. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-mediated diarrhea or colitis occurred in 7% (24/355) of patients receiving Opdualag, including Grade 3 (1.1%) and Grade 2 (4.5%) adverse reactions. Colitis led to permanent discontinuation of Opdualag in 2% and withholding of Opdualag in 2.8% of patients.
Immune-Mediated Hepatitis
Opdualag can cause immune-mediated hepatitis, defined as requiring the use of corticosteroids and no clear alternate etiology.
Immune-mediated hepatitis occurred in 6% (20/355) of patients receiving Opdualag, including Grade 4 (0.6%), Grade 3 (3.4%), and Grade 2 (1.4%) adverse reactions. Hepatitis led to permanent discontinuation of Opdualag in 1.7% and withholding of Opdualag in 2.3% of patients.
Immune-Mediated Endocrinopathies
Opdualag can cause primary or secondary adrenal insufficiency, hypophysitis, thyroid disorders, and Type 1 diabetes mellitus, which can be present with diabetic ketoacidosis. Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. In patients receiving Opdualag, adrenal insufficiency occurred in 4.2% (15/355) of patients receiving Opdualag, including Grade 3 (1.4%) and Grade 2 (2.5%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of Opdualag in 1.1% and withholding of Opdualag in 0.8% of patients.
Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Hypophysitis occurred in 2.5% (9/355) of patients receiving Opdualag, including Grade 3 (0.3%) and Grade 2 (1.4%) adverse reactions. Hypophysitis led to permanent discontinuation of Opdualag in 0.3% and withholding of Opdualag in 0.6% of patients.
Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Thyroiditis occurred in 2.8% (10/355) of patients receiving Opdualag, including Grade 2 (1.1%) adverse reactions. Thyroiditis did not lead to permanent discontinuation of Opdualag. Thyroiditis led to withholding of Opdualag in 0.3% of patients. Hyperthyroidism occurred in 6% (22/355) of patients receiving Opdualag, including Grade 2 (1.4%) adverse reactions. Hyperthyroidism did not lead to permanent discontinuation of Opdualag. Hyperthyroidism led to withholding of Opdualag in 0.3% of patients. Hypothyroidism occurred in 17% (59/355) of patients receiving Opdualag, including Grade 2 (11%) adverse reactions. Hypothyroidism led to the permanent discontinuation of Opdualag in 0.3% and withholding of Opdualag in 2.5% of patients.
Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated. Diabetes occurred in 0.3% (1/355) of patients receiving Opdualag, a Grade 3 (0.3%) adverse reaction, and no cases of diabetic ketoacidosis. Diabetes did not lead to the permanent discontinuation or withholding of Opdualag in any patient.
Immune-Mediated Nephritis with Renal Dysfunction
Opdualag can cause immune-mediated nephritis, which is defined as requiring use of steroids and no clear etiology. In patients receiving Opdualag, immune-mediated nephritis and renal dysfunction occurred in 2% (7/355) of patients, including Grade 3 (1.1%) and Grade 2 (0.8%) adverse reactions. Immune-mediated nephritis and renal dysfunction led to permanent discontinuation of Opdualag in 0.8% and withholding of Opdualag in 0.6% of patients.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
Immune-Mediated Dermatologic Adverse Reactions
Opdualag can cause immune-mediated rash or dermatitis, defined as requiring use of steroids and no clear alternate etiology. Exfoliative dermatitis, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and Drug Rash with eosinophilia and systemic symptoms has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.
Withhold or permanently discontinue Opdualag depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
Immune-mediated rash occurred in 9% (33/355) of patients, including Grade 3 (0.6%) and Grade 2 (3.4%) adverse reactions. Immune-mediated rash did not lead to permanent discontinuation of Opdualag. Immune-mediated rash led to withholding of Opdualag in 1.4% of patients.
Immune-Mediated Myocarditis
Opdualag can cause immune-mediated myocarditis, which is defined as requiring use of steroids and no clear alternate etiology. The diagnosis of immune-mediated myocarditis requires a high index of suspicion. Patients with cardiac or cardio-pulmonary symptoms should be assessed for potential myocarditis. If myocarditis is suspected, withhold dose, promptly initiate high dose steroids (prednisone or methylprednisolone 1 to 2 mg/kg/day) and promptly arrange cardiology consultation with diagnostic workup. If clinically confirmed, permanently discontinue Opdualag for Grade 2-4 myocarditis.
Myocarditis occurred in 1.7% (6/355) of patients receiving Opdualag, including Grade 3 (0.6%), and Grade 2 (1.1%) adverse reactions. Myocarditis led to permanent discontinuation of Opdualag in 1.7% of patients.
Other Immune-Mediated Adverse Reactions
The following clinically significant IMARs occurred at an incidence of <1% (unless otherwise noted) in patients who received Opdualag or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: Cardiac/Vascular: pericarditis, vasculitis; Nervous System: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; Ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other IMARs, consider a Vogt-Koyanagi-Harada–like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss; Gastrointestinal: pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis; Musculoskeletal and Connective Tissue: myositis/polymyositis, rhabdomyolysis (and associated sequelae including renal failure), arthritis, polymyalgia rheumatica; Endocrine: hypoparathyroidism; Other (Hematologic/Immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
Infusion-Related Reactions
Opdualag can cause severe infusion-related reactions. Discontinue Opdualag in patients with severe or life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild to moderate infusion-related reactions. In patients who received Opdualag as a 60-minute intravenous infusion, infusion-related reactions occurred in 7% (23/355) of patients.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 receptor blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 receptor blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and data from animal studies, Opdualag can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Opdualag for at least 5 months after the last dose of Opdualag.
Lactation
There are no data on the presence of Opdualag in human milk, the effects on the breastfed child, or the effect on milk production. Because nivolumab and relatlimab may be excreted in human milk and because of the potential for serious adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with Opdualag and for at least 5 months after the last dose.
Serious Adverse Reactions
In Relativity-047, fatal adverse reaction occurred in 3 (0.8%) patients who were treated with Opdualag; these included hemophagocytic lymphohistiocytosis, acute edema of the lung, and pneumonitis. Serious adverse reactions occurred in 36% of patients treated with Opdualag. The most frequent serious adverse reactions reported in ≥1% of patients treated with Opdualag were adrenal insufficiency (1.4%), anemia (1.4%), colitis (1.4%), pneumonia (1.4%), acute myocardial infarction (1.1%), back pain (1.1%), diarrhea (1.1%), myocarditis (1.1%), and pneumonitis (1.1%).
Common Adverse Reactions and Laboratory Abnormalities
The most common adverse reactions reported in ≥20% of the patients treated with Opdualag were musculoskeletal pain (45%), fatigue (39%), rash (28%), pruritus (25%), and diarrhea (24%).
The most common laboratory abnormalities that occurred in ≥20% of patients treated with Opdualag were decreased hemoglobin (37%), decreased lymphocytes (32%), increased AST (30%), increased ALT (26%), and decreased sodium (24%).
Please see US Full Prescribing Information for Opdualag.
Indication for OPDIVO® (nivolumab) + YERVOY® (ipilimumab)
OPDIVO, in combination with YERVOY, is indicated for the treatment of adult patients with unresectable or metastatic melanoma.
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO or YERVOY. Early identification and management are essential to ensure safe use of OPDIVO and YERVOY. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and periodically during treatment with OPDIVO and before each dose of YERVOY. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO or YERVOY interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
OPDIVO and YERVOY can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated pneumonitis occurred in 7% (31/456) of patients, including Grade 4 (0.2%), Grade 3 (2.0%), and Grade 2 (4.4%).
Immune-Mediated Colitis
OPDIVO and YERVOY can cause immune-mediated colitis, which may be fatal. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated colitis occurred in 25% (115/456) of patients, including Grade 4 (0.4%), Grade 3 (14%) and Grade 2 (8%).
Immune-Mediated Hepatitis and Hepatotoxicity
OPDIVO and YERVOY can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated hepatitis occurred in 15% (70/456) of patients, including Grade 4 (2.4%), Grade 3 (11%), and Grade 2 (1.8%).
Immune-Mediated Endocrinopathies
OPDIVO and YERVOY can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, adrenal insufficiency occurred in 8% (35/456), including Grade 4 (0.2%), Grade 3 (2.4%), and Grade 2 (4.2%).
In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hypophysitis occurred in 9% (42/456), including Grade 3 (2.4%) and Grade 2 (6%).
In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hyperthyroidism occurred in 9% (42/456) of patients, including Grade 3 (0.9%) and Grade 2 (4.2%).
In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, hypothyroidism occurred in 20% (91/456) of patients, including Grade 3 (0.4%) and Grade 2 (11%).
In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
OPDIVO and YERVOY can cause immune-mediated nephritis. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
YERVOY can cause immune-mediated rash or dermatitis, including bullous and exfoliative dermatitis, SJS, TEN, and DRESS. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes.
Withhold or permanently discontinue OPDIVO and YERVOY depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%). In patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, immune-mediated rash occurred in 28% (127/456) of patients, including Grade 3 (4.8%) and Grade 2 (10%).
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or OPDIVO in combination with YERVOY or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
In addition to the immune-mediated adverse reactions listed above, across clinical trials of YERVOY monotherapy or in combination with OPDIVO, the following clinically significant immune-mediated adverse reactions, some with fatal outcome, occurred in <1% of patients unless otherwise specified: nervous system: autoimmune neuropathy (2%), myasthenic syndrome/myasthenia gravis, motor dysfunction; cardiovascular: angiopathy, temporal arteritis; ocular: blepharitis, episcleritis, orbital myositis, scleritis; gastrointestinal: pancreatitis (1.3%); other (hematologic/immune): conjunctivitis, cytopenias (2.5%), eosinophilia (2.1%), erythema multiforme, hypersensitivity vasculitis, neurosensory hypoacusis, psoriasis.
Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO and YERVOY, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
OPDIVO and YERVOY can cause severe infusion-related reactions. Discontinue OPDIVO and YERVOY in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO. In melanoma patients receiving OPDIVO 1 mg/kg with YERVOY 3 mg/kg every 3 weeks, infusion-related reactions occurred in 2.5% (10/407) of patients.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO or YERVOY. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO or YERVOY and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO and YERVOY prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, OPDIVO and YERVOY can cause fetal harm when administered to a pregnant woman. The effects of YERVOY are likely to be greater during the second and third trimesters of pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and YERVOY and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
There are no data on the presence of OPDIVO or YERVOY in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
In Checkmate 067, serious adverse reactions (74% and 44%), adverse reactions leading to permanent discontinuation (47% and 18%) or to dosing delays (58% and 36%), and Grade 3 or 4 adverse reactions (72% and 51%) all occurred more frequently in the OPDIVO plus YERVOY arm (n=313) relative to the OPDIVO arm (n=313). The most frequent (≥10%) serious adverse reactions in the OPDIVO plus YERVOY arm and the OPDIVO arm, respectively, were diarrhea (13% and 2.2%), colitis (10% and 1.9%), and pyrexia (10% and 1.0%).
Common Adverse Reactions
In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO plus YERVOY arm (n=313) were fatigue (62%), diarrhea (54%), rash (53%), nausea (44%), pyrexia (40%), pruritus (39%), musculoskeletal pain (32%), vomiting (31%), decreased appetite (29%), cough (27%), headache (26%), dyspnea (24%), upper respiratory tract infection (23%), arthralgia (21%), and increased transaminases (25%). In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO arm (n=313) were fatigue (59%), rash (40%), musculoskeletal pain (42%), diarrhea (36%), nausea (30%), cough (28%), pruritus (27%), upper respiratory tract infection (22%), decreased appetite (22%), headache (22%), constipation (21%), arthralgia (21%), and vomiting (20%).
Please see US Full Prescribing Information for OPDIVO and YERVOY.
References
1. Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long-term survival results on metastatic melanoma treatment. J Immunother Cancer. 2020. doi:10.1136/jitc-2020-000948.
2. Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384(23):2229-2240.
3. OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34.
5. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546.
6. Cancer Research Institute. FDA Approval Timeline of Active Immunotherapies. Updated June 27, 2022. Accessed July 11, 2022. https://www.cancerresearch.org/en-us/scientists/immuno-oncology-landscape/fda-approval-timeline-of-active-immunotherapies.
7. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34.
8. Opdualag [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
9. PubD 00058298. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
10. Long GV, Hodi FS, Lipson EJ, et al. Relatlimab and nivolumab vs nivolumab in previously untreated metastatic or unresectable melanoma: overall survival and response rates from RELATIVITY-047 (CA224-047). Oral presentation at ASCO Plenary Series 2022. Presentation number 9505.
11. US Food and Drug Administration. CFR–Code of Federal Regulations Title 21. Updated March 29, 2022. Accessed July 1, 2022.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=300.50.
© 2022 Bristol-Myers Squibb Company. OPDIVO®, YERVOY®, Opdualag™, and the related logos are trademarks of Bristol-Myers Squibb Company.
7356-US-2200441 8/22
Additional Definitions
AJCC=American Joint Committee on Cancer; BICR=blinded independent central review; CI=confidence interval;CTLA-4=cytotoxic T-lymphocyte antigen 4; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; HR=hazard ratio;IHC=immunohistochemistry; IV=intravenous;LAG-3=lymphocyte-activation gene 3; LDH=lactate dehydrogenase; M stage=metastasis stage; mo=month; no=number; NS=not significant; PD-1=programmed death receptor-1; PD-L1=programmed death ligand 1; q4w=every 4 weeks; RECIST=Response Evaluation Criteria In Solid Tumors.
Late Breaking Abstract – ASCO 2022: Improved Distant Metastasis-Free Survival with Adjuvant KEYTRUDA® in High Risk Stage II Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2022, about 99,780 new cases of melanoma of the skin will be diagnosed in the United States and 7,650 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma, with a 5-year survival rate of 98% for Stage I disease and 90% for Stage II disease. The current standard of care for patients following resection of high-risk Stage II disease is observation, even though patients with Stage IIB and IIC disease presenting with high-risk features (depth of invasion, T-category, ulceration) have 5 and 10 year melanoma-specific survival similar to that of patients with Stage IIIA and IIIB disease.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response. The FDA in 2019, approved KEYTRUDA® for the adjuvant treatment of patients with melanoma, with involvement of lymph node(s), following complete resection (Stage III). The present study was conducted to evaluate the role of adjuvant immunotherapy in patients with high risk Stage II melanoma.
KEYNOTE-716 is a randomized, double-blind, Phase III trial, in which 976 patients aged 12 years or older, with completely resected cutaneous Stage IIB or IIC melanoma, and no lymph node involvement, were randomly assigned 1:1 to receive KEYTRUDA® 200 mg IV (2 mg/kg for pediatric patients) or placebo, every 3 weeks for 17 cycles (up to 1 year). Patients were stratified by T category 3b, 4a, 4b (adults) and with a separate stratum for pediatric patients. Approximately 65% had Stage IIB disease and 35% had Stage IIC disease. There was no prespecified analysis for PD-L1 or BRAF status in this study, as there was inconsistent and small amounts of tissue available for testing. This was the first part (Part 1) of this double-blind study. The Primary endpoint was Relapse Free Survival (RFS) per investigator assessment, and Safety. The second part (Part 2) of this study was open-label design, and adults and pediatric patients were eligible to receive up to 35 additional cycles of treatment, only if they had recurrence after receiving the placebo or completed 17 cycles of KEYTRUDA®. Patients in the KEYTRUDA® group who experienced disease recurrence within 6 months of completing the treatment were excluded from Part 2 of the study. Secondary end points included Distant Metastasis Free Survival (DMFS), Overall Survival (OS) and Quality of Life.
At median follow up of 14.4 months, adjuvant KEYTRUDA® significantly prolonged RFS compared to placebo (HR=0.65; P=0.00658), in patients with resected Stage IIB or IIC melanoma. At the time of this analysis, 11.1% of patients on KEYTRUDA® had a recurrence, compared to 16.8% of those receiving placebo. The 12-month RFS rate was 90.5% for KEYTRUDA® versus 83.1% for placebo.
The researchers herein presented new data from the analysis of Distant Metastasis-Free Survival (DMFS) and Recurrence Free Survival (RFS), with a longer median follow up of 26.9 months. Adjuvant KEYTRUDA® significantly improved DMFS when compared to placebo (HR=0.64; P=0.0029), representing a 36% reduction in the risk of recurrence. The 24-month DMFS rate was 88.1% versus 82.2%, respectively. Grade 3 or more Adverse Events occurred in 28.4% of patients in the KEYTRUDA® group, versus 20% in the placebo group. Hypothyroidism was the most common immune mediated Adverse Event with KEYTRUDA®, compared to placebo (17.2% versus 3.7%).
The authors concluded that adjuvant KEYTRUDA® for resected Stage IIB and IIC melanoma, significantly improved Distant Metastasis-Free Survival, with continued reduction in the risk of recurrence, and a favorable benefit-risk profile. KEYNOTE-716 is the first randomized Phase III trial of an anti-PD-1 therapy in resected Stage II melanoma, and these findings represent an important milestone for this patient group.
Distant metastasis-free survival with pembrolizumab versus placebo as adjuvant therapy in stage IIB or IIC melanoma: The phase 3 KEYNOTE-716 study. Long GV, Luke JJ, Khattak M, et al. DOI: 10.1200/JCO.2022.40.17_suppl.LBA9500 Journal of Clinical Oncology 40, no. 17_suppl (June 10, 2022) LBA9500-LBA9500.
OPDUALAG® (Nivolumab and Relatlimab-rmbw)
The FDA on March 18, 2022, approved OPDUALAG® for adult and pediatric patients 12 years of age or older with unresectable or metastatic melanoma. OPDUALAG® is a fixed-dose combination of the LAG-3-blocking antibody Relatlimab and the Programmed Death receptor-1 blocking antibody, Nivolumab. OPDUALAG® is a product of Bristol-Myers Squibb Company.
