The FDA on December 3,2021, approved KEYTRUDA® for the adjuvant treatment of adult and pediatric (≥12 years of age) patients with Stage IIB or IIC melanoma following complete resection. KEYTRUDA® is a product of Merck.
Tag: Malignant Melanoma of the Skin
FDA Approves LAG-3 Inhibitor OPDUALAG® and OPVIDO® in Advanced Untreated Melanoma
SUMMARY: The FDA on March 18, 2022, approved OPDIVO® (Nivolumab) and OPDUALAG® (Relatlimab-rmbw), for adult and pediatric patients 12 years of age or older, with unresectable or metastatic melanoma. The American Cancer Society’s estimates that for 2022, about 99,780 new cases of melanoma of the skin will be diagnosed in the United States and 7,650 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
A better understanding of Immune checkpoints has opened the doors for the discovery of novel immune targets. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By blocking the Immune checkpoint proteins, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.
YERVOY® (Ipilimumab), a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4 was compared with PD-1 inhibitors, OPDIVO® (Nivolumab) and KEYTRUDA® (Pembrolizumab) in patients with advanced melanoma, and both OPDIVO® and KEYTRUDA® have demonstrated superior Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR), and with a better safety profile. In the CheckMate 067, which is a double-blind Phase III study, results from the 6.5 year analysis showed that a combination of OPDIVO® plus YERVOY® demonstrated significant improvement in OS and PFS, when compared to single agent OPDIVO® or single agent YERVOY®.
In an attempt to improve outcomes and enhance the risk-benefit profiles of immunotherapy combinations, alternate Immune checkpoints are being explored. LAG-3 (Lymphocyte-Activation Gene 3 (LAG-3), is a cell-surface receptor expressed on immune cells including activated CD4+ T cells, and negatively regulates T-cell proliferation, inhibits T-cell activation and effector T-cell function. LAG-3 is upregulated in several tumor types, including malignant melanoma.
OPDUALAG® (Relatlimab) is a first-in-class human IgG4 LAG-3–blocking antibody that binds to LAG-3 and restores the effector function of exhausted T cells, resulting in T cell proliferation, activation and a therapeutic response. In preclinical studies, dual inhibition of LAG-3 and PD-1 showed synergistic antitumor activity, and in a Phase I/II trial, the combination of OPDUALAG® and OPDIVO®, demonstrated durable Objective Responses in patients with Relapsed/Refractory melanoma following treatment with PD-1 inhibitors.
RELATIVITY-047 is a Phase II/III, global, multicenter, double-blind, randomized trial in which a fixed-dose combination of OPDUALAG® and OPDIVO® was compared with OPDIVO® alone, in patients with previously untreated metastatic or unresectable melanoma. In this study, 714 patients were randomly assigned 1:1 to receive OPDUALAG® 160 mg and OPDIVO® 480 mg in a fixed-dose combination (N=355) or single agent OPDIVO® 480 mg (N=359). Both regimens were administered as an IV infusion over 60 minutes every 4 weeks, and treatment was continued until disease progression, unacceptable toxicities, or withdrawal of consent. Both treatment groups were well balanced and patients were stratified according to LAG-3 expression (1% or more versus less than 1%), PD-L1 expression (1% or more versus less than 1%), BRAF V600 mutation status, and metastasis stage (M0 or M1 with normal LDH levels versus M1 with elevated LDH levels). More patients in the OPDUALAG®- OPDIVO® group had Stage M1c disease, and a larger proportion had three or more sites with at least one metastatic lesion. The Primary end point was Progression Free Survival (PFS) as assessed by blinded Independent Central Review. Secondary end points included Overall Survival and Objective Response Rate (ORR). The median follow up was 13.2 months and the use of subsequent therapies upon progression was similar in the two treatment groups.
The median PFS was 10.1 months with OPDUALAG®- OPDIVO® as compared with 4.6 months with OPDIVO® (HR=0.75; P=0.006). The PFS benefit at 12 months with OPDUALAG®- OPDIVO® was 47.7% compared to 36.0% with OPDIVO®. The PFS benefit was more so with Relatlimab- OPDIVO® across key prespecified subgroups, compared to single agent OPDIVO®. Patients with poor prognosis characteristics, such as visceral metastases, high tumor burden, elevated levels of serum LDH, or mucosal or acral melanoma, had better outcomes with OPDUALAG®- OPDIVO® combination, than with single agent OPDIVO®. Further, a benefit with OPDUALAG®- OPDIVO® was also noted across BRAF mutant and wild-type subgroups, compared to OPDIVO®. Expression of LAG-3 or PD-L1 was not useful in predicting a benefit of OPDUALAG®- OPDIVO® over single agent OPDIVO® and appears to NOT have a clear role in treatment selection.
Grade 3 or 4 toxicities occurred in 18.9% of patients in the OPDUALAG®- OPDIVO® group and in 9.7% of patients in the single agent OPDIVO® group. The Safety profile of OPDUALAG®- OPDIVO® appeared favorable, when compared with dual checkpoint inhibition with a CTLA-4 inhibitor and PD-1 inhibitor combination (YERVOY®- OPDIVO®) in the CheckMate 067 trial, in which Adverse Events were noted in 59% of patients.
It was concluded that inhibition of two immune checkpoints, LAG-3 and PD-1, provided greater benefit with regards to Progression Free Survival, than inhibition of PD-1 alone, in patients with previously untreated metastatic or unresectable melanoma. The authors added that these results validate blocking LAG-3 in combination with PD-1 as a therapeutic strategy for patients with melanoma, and establishes LAG-3 as the third immune checkpoint pathway, thus providing more treatment options for patients with advanced melanoma.
Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. Tawbi HA, Schadendorf D, Lipson EJ, et al. for the RELATIVITY-047 Investigators. N Engl J Med 2022;386:24-34.
Association of Gut Microbiome with Immune Checkpoint Inhibitor Response in Advanced Melanoma
SUMMARY: The American Cancer Society estimates that in 2022, there will be an estimated 1.92 million new cancer cases diagnosed and 609,360 cancer deaths in the United States. Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate and prolongation of survival across multiple tumor types. These agents target Programmed cell Death protein-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1), Cytotoxic T-Lymphocyte-Associated protein-4 (CTLA-4), and many other important regulators of the immune system. Over 50% of patients treated with a combination of PD-1 and CTLA-4 inhibitors are alive after five years. Nonetheless, less than 50% of the patients respond to single-agent ICI and a higher response to targeting both PD-1 and CTLA-4 is associated with significant immune-related Adverse Events.
Biomarkers predicting responses to ICI’s include Tumor Mutational Burden (TMB), Mismatch Repair (MMR) status, and Programmed cell Death Ligand 1 (PD‐L1) expression. Other biomarkers such as Tumor Infiltrating Lymphocytes (TILs), TIL‐derived Interferon‐γ, Neutrophil‐to‐Lymphocyte ratio, and peripheral cytokines, have also been proposed as predictors of response. It has been postulated that concomitant medications during therapy with ICIs such as baseline steroid use as well as treatment with antibiotics may negate or lessen the efficacy of ICIs.
Preclinical studies have suggested that immune-based therapies for cancer may have a very complex interplay with the host’s microbiome and there may be a relationship between gut bacteria and immune response to cancer. The gut microbiome is unique in each individual, including identical twins. The crosstalk between microbiota in the gut and the immune system allows for the tolerance of commensal bacteria (normal microflora) and oral food antigens and at the same time enables the immune system to recognize and attack opportunistic bacteria. Immune Checkpoint Inhibitors strongly rely on the influence of the host’s microbiome, and the gut microbial diversity enhances mucosal immunity, dendritic cell function, and antigen presentation. Broad-spectrum antibiotics can potentially alter the bacterial composition and diversity of our gut microbiota, by killing the good bacteria. It has been postulated that this may negate the benefits of immunotherapy and influence treatment outcomes. It should be noted however that the relationship between gut bacteria and immune response is influenced by several factors and may be partially cancer type specific and it is unlikely that the same microbiome features can reflect the uniqueness of the genetic and immune characteristics of each tumor.
Even though the composition of the gut microbiome has been associated with clinical responses to immune checkpoint inhibitor (ICI) treatment, there is a lack of consistency of results between the published studies, and there is limited consensus on the specific microbiome characteristics linked to the clinical benefits of ICIs. The Predicting Response to Immunotherapy for Melanona with Gut Microbiome and Metabolomics (PRIMM) studies are two separate prospective observational cohort studies that has been recruiting patients in the UK (PRIMM-UK) and the Netherlands (PRIMM-NL) since 2018. These cohorts of previously ICI-naive patients with advanced melanoma have provided extensive biosamples, including stool, serum and peripheral blood mononuclear cells, before and during ICI treatment, with detailed clinical and dietary data collected at regular intervals longitudinally.
The authors therefore performed a meta-analysis on existing publicly available datasets to produce the largest study to date. In order to study the role of the gut microbiome in ICI response, the researchers recruited ICI-naive patients with advanced cutaneous melanoma from the PRIMM cohorts, as well as three additional cohorts of ICI-naive patients with advanced cutaneous melanoma, originating from Barcelona, Leeds and Manchester (N = 165), and performed shotgun metagenomic sequencing on a total of 165 stool microbiome samples collected before initiating ICI treatment. Shotgun sequencing is a laboratory technique for determining the DNA sequence of an organism’s genome. This dataset was integrated with 147 metagenomic samples from smaller publicly available datasets. This methodology provided the largest assessment of the potential of the gut microbiome as a biomarker of response to ICI, in addition to allowing for investigation of specific microbial species or functions associated with response. Patient demographics including age, gender, BMI, previous non-immunotherapy treatments, previous drug therapies such as antibiotics, Proton Pump Inhibitors (PPIs) and steroids, as well as dietary patterns, were collected in these cohorts for the majority of patients, and were considered in the multivariate analysis.
The researchers used machine learning analysis to understand the association between gut microbiome and response to ICIs. This analysis confirmed the link between the microbiome and Overall Response Rates (ORRs), as well as Progression Free Survival (PFS) with ICIs. This analysis also revealed limited reproducibility of microbiome-based signatures across cohorts. A panel of species, including Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansiamuciniphila were associated with responders, but no single species could be regarded as a fully reliable biomarker across studies. Based on these findings from this large set of real-world cohorts, the authors noted that the relationship between human gut microbiome and response to ICIs is more complex than previously understood, and extends beyond the presence or absence of different microbial species in responders and nonresponders.
It was concluded that future studies should include large samples and take into account the complex interplay of clinical factors with the gut microbiome over the treatment course. Until then, the authors recommend high-quality, diverse, whole-foods diet to optimize gut health, rather than consumption of commercial probiotics.
Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Lee KA, Thomas AM, Bolte LA, et al. Nat Med. 2022 Feb 28. doi: 10.1038/s41591-022-01695-5. Online ahead of print.
LAG-3 Inhibitor Relatlimab and OPVIDO® in Advanced Untreated Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2022, about 99,780 new cases of melanoma of the skin will be diagnosed in the United States and 7,650 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
A better understanding of Immune checkpoints has opened the doors for the discovery of novel immune targets. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By blocking the Immune checkpoint proteins, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.
YERVOY® (Ipilimumab), a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4 was compared with PD-1 inhibitors, OPDIVO® (Nivolumab) and KEYTRUDA® (Pembrolizumab) in patients with advanced melanoma, and both OPDIVO® and KEYTRUDA® have demonstrated superior Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR), and with a better safety profile. In the CheckMate 067, which is a double-blind Phase III study, results from the 6.5 year analysis showed that a combination of OPDIVO® plus YERVOY® demonstrated significant improvement in OS and PFS, when compared to single agent OPDIVO® or single agent YERVOY®.
In an attempt to improve outcomes and enhance the risk-benefit profiles of immunotherapy combinations, alternate Immune checkpoints are being explored. LAG-3 (Lymphocyte-Activation Gene 3 (LAG-3), is a cell-surface receptor expressed on immune cells including activated CD4+ T cells, and negatively regulates T-cell proliferation, inhibits T-cell activation and effector T-cell function. LAG-3 is upregulated in several tumor types, including malignant melanoma.
Relatlimab is a first-in-class human IgG4 LAG-3–blocking antibody that binds to LAG-3 and restores the effector function of exhausted T cells, resulting in T cell proliferation, activation and a therapeutic response. In preclinical studies, dual inhibition of LAG-3 and PD-1 showed synergistic antitumor activity, and in a Phase I/II trial, the combination of Relatlimab and Nivolumab, demonstrated durable Objective Responses in patients with Relapsed/Refractory melanoma following treatment with PD-1 inhibitors.
RELATIVITY-047 is a Phase II/III, global, multicenter, double-blind, randomized trial in which a fixed-dose combination of Relatlimab and Nivolumab was compared with Nivolumab alone, in patients with previously untreated metastatic or unresectable melanoma. In this study, 714 patients were randomly assigned 1:1 to receive Relatlimab 160 mg and Nivolumab 480 mg in a fixed-dose combination (N=355) or single agent Nivolumab 480 mg (N=359). Both regimens were administered as an IV infusion over 60 minutes every 4 weeks, and treatment was continued until disease progression, unacceptable toxicities, or withdrawal of consent. Both treatment groups were well balanced and patients were stratified according to LAG-3 expression (1% or more versus less than 1%), PD-L1 expression (1% or more versus less than 1%), BRAF V600 mutation status, and metastasis stage (M0 or M1 with normal LDH levels versus M1 with elevated LDH levels). More patients in the Relatlimab-Nivolumab group had Stage M1c disease, and a larger proportion had three or more sites with at least one metastatic lesion. The Primary end point was Progression Free Survival (PFS) as assessed by blinded Independent Central Review. Secondary end points included Overall Survival and Objective Response Rate (ORR). The median follow up was 13.2 months and the use of subsequent therapies upon progression was similar in the two treatment groups.
The median PFS was 10.1 months with Relatlimab-Nivolumab as compared with 4.6 months with Nivolumab (HR=0.75; P=0.006). The PFS benefit at 12 months with Relatlimab-Nivolumab was 47.7% compared to 36.0% with Nivolumab. The PFS benefit was more so with Relatlimab- Nivolumab across key prespecified subgroups, compared to single agent Nivolumab. Patients with poor prognosis characteristics, such as visceral metastases, high tumor burden, elevated levels of serum LDH, or mucosal or acral melanoma, had better outcomes with Relatlimab-Nivolumab combination, than with single agent Nivolumab. Further, a benefit with Relatlimab-Nivolumab was also noted across BRAF mutant and wild-type subgroups, compared to Nivolumab. Expression of LAG-3 or PD-L1 was not useful in predicting a benefit of Relatlimab-Nivolumab over single agent Nivolumab and appears to NOT have a clear role in treatment selection.
Grade 3 or 4 toxicities occurred in 18.9% of patients in the Relatlimab-Nivolumab group and in 9.7% of patients in the single agent Nivolumab group. The Safety profile of Relatlimab-Nivolumab appeared favorable, when compared with dual checkpoint inhibition with a CTLA-4 inhibitor and PD-1 inhibitor combination (Ipilimumab-Nivolumab) in the CheckMate 067 trial, in which Adverse Events were noted in 59% of patients.
It was concluded that inhibition of two immune checkpoints, LAG-3 and PD-1, provided greater benefit with regards to Progression Free Survival, than inhibition of PD-1 alone, in patients with previously untreated metastatic or unresectable melanoma. The authors added that these results validate blocking LAG-3 in combination with PD-1 as a therapeutic strategy for patients with melanoma, and establishes LAG-3 as the third immune checkpoint pathway, thus providing more treatment options for patients with advanced melanoma.
Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. Tawbi HA, Schadendorf D, Lipson EJ, et al. for the RELATIVITY-047 Investigators. N Engl J Med 2022;386:24-34.
Late Breaking Abstract – ESMO 2021: Adjuvant KEYTRUDA® for High Risk Stage II Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2021, about 106,110 new cases of melanoma will be diagnosed in the United States and 7,180 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
Surgical resection with a curative intent is the standard of care for patients with early stage melanoma, with a 5-year survival rate of 98% for Stage I disease and 90% for Stage II disease. The current standard of care for patients following resection of high-risk Stage II disease is observation, even though patients with Stage IIB and IIC disease presenting with high-risk features (depth of invasion, T-category, ulceration) have 5 and 10 year melanoma-specific survival similar to that of patients with Stage IIIA and IIIB disease.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. By doing so, it unleashes the tumor-specific effector T cells, and is thereby able to undo PD-1 pathway-mediated inhibition of the immune response. The FDA in 2019, approved KEYTRUDA® for the adjuvant treatment of patients with melanoma, with involvement of lymph node(s) following complete resection (Stage III). The present study was conducted to evaluate the role of adjuvant immunotherapy in patients with high risk Stage II melanoma.
KEYNOTE-716 is a randomized, double-blind, Phase III trial, in which 976 patients aged 12 years or older, with completely resected cutaneous Stage IIB or IIC melanoma, and no lymph node involvement, were randomly assigned 1:1 to receive KEYTRUDA® 200 mg (2 mg/kg for pediatric patients) or placebo, every 3 weeks for 17 cycles (up to 1 year). Patients were stratified by T category 3b, 4a, 4b (adults) and with a separate stratum for pediatric patients. Approximately 65% had Stage IIB disease and 35% had Stage IIC disease. There was no prespecified analysis for PD-L1 or BRAF status in this study, as there was inconsistent and small amounts of tissue available for testing. This was the first part (Part 1) of this double-blind study. The Primary endpoint was Relapse Free Survival (RFS) per investigator assessment, and Safety. The second part (Part 2) of this study was open-label design, and adults and pediatric patients were eligible to receive up to 35 additional cycles of treatment, only if they had recurrence after receiving the placebo or completed 17 cycles of KEYTRUDA®. Patients in the KEYTRUDA® group who experienced disease recurrence within 6 months of completing the treatment were excluded from Part 2 of the study. Secondary end points included Distant Metastasis-Free Survival, Overall Survival (OS) and Quality of Life. The researchers herein reported the results at the interim analysis of Part 1 of this study, and Part 2 data are not yet mature.
At median follow up of 14.4 months, the study met its Primary end point of RFS at the first protocol-specified analysis. KEYTRUDA® significantly prolonged RFS compared to placebo (HR=0.65; P=0.00658). At the time of this analysis, 11.1% of patients on KEYTRUDA® had a recurrence, compared to 16.8% of those receiving placebo. The 12-month RFS rate was 90.5% for KEYTRUDA® versus 83.1% for placebo. Median RFS was Not Reached in either group at the time of this analysis. Quality of Life scores were similar between the KEYTRUDA® and placebo groups at all time points.
It was concluded that adjuvant KEYTRUDA® for resected Stage IIB and IIC melanoma decreased the risk of disease recurrence or death by 35% compared with placebo, and was associated with significantly prolonged Relapse Free Survival and a favorable benefit-risk profile. KEYNOTE-716 is the first randomized Phase III trial of an anti-PD-1 therapy in resected Stage II melanoma, and these findings represent an important milestone for this patient group.
LBA3_PR – Pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. Luke JJ, Rutkowski P, Queirolo P, et al. Annals of Oncology (2021) 32 (suppl_5): S1283-S1346. 10.1016/annonc/annonc741.
Long Term Survival Benefit in Advanced Melanoma with OPDIVO® plus YERVOY®
SUMMARY: The American Cancer Society’s estimates that for 2021, about 106,110 new cases of melanoma will be diagnosed in the United States and 7,180 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
A better understanding of Immune checkpoints has opened the doors for the discovery of novel immune targets. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By blocking the Immune checkpoint proteins, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.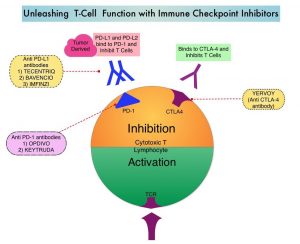
YERVOY® (Ipilimumab) is a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4, and was the first systemic therapy in randomized Phase III trials, to show prolonged Overall Survival (OS) in patients with advanced melanoma. YERVOY® in a pooled analysis of data from 12 studies showed a 3-year Overall Survival of 26% among treatment naive patients, and survival up to 10 years in approximately 20% of all patients, with advanced melanoma. The two PD-1 inhibitors of interest are OPDIVO® (Nivolumab) and KEYTRUDA® (Pembrolizumab), which are fully human, Immunoglobulin G4, anti-PD-1 targeted monoclonal antibodies that bind to the PD-1 receptor, and block its interaction with ligands PD-L1 and PD-L2, following which the tumor-specific effector T cells are unleashed. They are thus able to undo PD-1 pathway-mediated inhibition of the immune response. When compared with YERVOY® in patients with advanced melanoma, PD-1 inhibitors, both OPDIVO® and KEYTRUDA® have demonstrated superior Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR), with a better safety profile. OPDIVO® in combination with YERVOY® in a Phase I study resulted in an Overall Survival of 68% at 3 years among patients with advanced melanoma, regardless of prior therapies.
CheckMate 067 is a double-blind Phase III study in which patients with previously untreated advanced melanoma were randomly assigned in a 1:1:1 ratio to receive one of the three regimens: OPDIVO® 1 mg/kg every 3 weeks plus YERVOY® 3 mg/kg every 3 weeks for four doses, followed by OPDIVO® 3 mg/kg every 2 weeks (N=314); OPDIVO® 3 mg/kg every 2 weeks plus placebo (N=316); or YERVOY® 3 mg/kg every 3 weeks for four doses plus placebo (N=315). Randomization was stratified according to BRAF mutation status, metastasis stage, and Programmed cell Death Ligand 1 (PD-L1) status. Treatment was continued until disease progression or unacceptable toxicities. The two Primary end points were PFS and OS in the OPDIVO® plus YERVOY® group, and in the OPDIVO® group versus the YERVOY® group.
As previously reported, there was a durable and sustained clinical benefit at 5 years, with superior PFS and OS among patients treated with OPDIVO® plus YERVOY® combination therapy or with OPDIVO® alone, compared with single agent YERVOY®. The authors in this publication reported the efficacy and safety outcomes in this untreated, unresectable Stage III or IV patients with advanced melanoma, after an extended follow up of 6.5 years.
The median Overall Survival for patients treated with OPDIVO® plus YERVOY® combination therapy was 72.1 months, for those treated with single agent OPDIVO® was 36.9 months, compared with 19.9 months with single agent YERVOY®. At the time of analysis at 6.5 years, 49% of patients treated with OPDIVO® plus YERVOY® were alive, compared to 42% of those treated with OPDIVO® alone and 23% of those treated with single agent YERVOY®. The PFS at 6.5 years was 34% for the OPDIVO® plus YERVOY® group, 29% for the OPDIVO® alone group, and 7% for the YERVOY® group.
It was concluded that the results from the 6.5 year analysis showed durable improved outcomes with OPDIVO® plus YERVOY®, and OPDIVO® alone, when compared to single agent YERVOY®, among patients with advanced melanoma. Further, there was an improvement in OS and PFS with OPDIVO® plus YERVOY®, over OPDIVO® alone. The authors added that this analysis represents the longest follow up from a Phase III melanoma trial in the modern checkpoint inhibitor combination therapy and targeted therapy era.
CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. J Clin Oncol 39, 2021 (suppl 15; abstr 9506)
American Society of Clinical Oncology Policy Statement on Skin Cancer Prevention
SUMMARY: Skin Cancer is the most common cancer diagnosed in the US and around the world. Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC) are the two most common types of skin cancers. It is estimated that 5.4 million cases of BCC and SCC are diagnosed each year in the US (occurring in about 3.3 million Americans, as some individuals have more than one type of skin cancer), and 8 of 10 are BCCs, whereas SCCs occur less often. Although the overall mortality rate from these cancers are low, SCCs are almost exclusively responsible for approximately 3,000 deaths per year in the US, with the greatest mortality risk among transplant recipients, who are immunocompromised. Malignant Melanoma of the skin occurs less frequently than BCC and SCC, and the American Cancer Society estimates that in the US for 2020, about 100,350 new melanomas will be diagnosed and about 6,850 people are expected to die of the disease. The rates of skin cancer have been rising rapidly over the past several years, with the economic cost estimates of $8.1 billion annually in the US.
Exposure to UltraViolet (UV) rays is a major risk factor for most skin cancers. Sunlight is the main source of UV rays. UV rays-emitting indoor tanning devices such as tanning beds, sunlamps, and UV lamps, are another source of UV rays. The risk of UV rays associated skin cancers, particularly for SCC, is dose dependent, and increases with greater duration and intensity of exposure. This risk is increased with cumulative solar UV rays exposure over an individual’s lifetime. For a given level of UV rays exposure, skin cancer risk is highest among UV ray sensitive phenotypes who typically are fair skinned, and have a propensity to sunburn, blister and/or freckle, upon exposure to UV rays. National surveys on sun exposure in the US illustrate the high rates of sunburn among adults (35%) and high school students (57%), emphasizing the importance of primary and secondary prevention strategies in the younger population. The ASCO’s 2019 National Cancer Opinion Survey found that only 49% of respondents reported using sunscreen to prevent skin cancer. Skin cancer is less common in individuals with darker skin colors (Black and Latino individuals), due to greater levels of melanin in the skin, which inherently has photoprotective ability. Nonetheless, when skin cancers do occur in individuals with darker skin tones, they tend to be more aggressive, possibly due to delayed diagnosis, as these individuals may be less aware of their skin cancer risks.
Given that skin cancer has such a major impact on society, the American Society of Clinical Oncology (ASCO) earlier this year published a policy statement aimed at lessening the burden of skin cancer through reducing exposure to UV radiation for youth and adults. This policy statement included a review of the risk factors for skin cancer, disparities in incidence, diagnosis and survival among different populations, and public health strategies for Primary and Secondary skin cancer prevention.
ASCO presented recommendations across the following four themes:
Reduce Exposure to Indoor Tanning
1) A major opportunity to prevent skin cancer is by reducing UV ray exposure through avoidance of indoor tanning.
2) Avoidance of indoor tanning holds particular promise in influencing adolescents and sexual minority men because of their higher rate of exposure to tanning.
3) The International Agency for Research on Cancer concluded that UV ray-emitting indoor tanning devices were carcinogens and there is now high-quality scientific evidence documenting strong and consistent associations between indoor tanning devices and skin cancer risk.
4) Indoor tanning is higher among non-Hispanic white females compared with all other population subgroups, and its direct association with melanoma risk likely explains the higher melanoma incidence in this group compared with male adolescents and young adults.
5) There is evidence suggesting the presence of “tanning dependence”, similar to substance use dependence, among individuals who engage in indoor tanning.
6) Recognizing that indoor tanning devices are a threat to public health, several cancer care and public health organizations support strong restrictions designed to prevent the use of UV ray-emitting tanning devices. ASCO supports strengthened laws and regulations restricting such products
Increase Public Efforts to Promote Sun Protection
1) Local, state, and federal laws should support policies that allow students to carry and use sunscreen products without physician authorization.
2) Enhance the protection of young people by encouraging the increased use of broad-spectrum sunscreen and protective clothing, through educational programs.
3) Improvement in sunscreen products and public education to prevent intentional sun exposure, for promoting Vitamin D synthesis.
4) Private and public institutions should be encouraged in their efforts to create more shaded areas in places used for outdoor recreation.
5) Development of new educational efforts, to change the social perceptions of tanned skin.
Community Education and Outreach
1) Investing in prevention research, and continued support for the National Cancer Institute’s Division of Cancer Prevention and the Centers for Disease Control and Prevention’s National Skin Cancer Prevention Education Program, to address the burden of this disease among persons of color, lower socioeconomic status populations, and sexual minorities.
2) ASCO and the cancer care community should work together to develop effective methods for outreach and health communication, to the diverse segments of the population at risk of skin cancer.
Role of Oncology Providers
1) Research has shown that cancer survivors do not adhere to skin-protective behaviors, anymore than the lay public who have not been diagnosed with cancer.
2) Oncologists should discuss with their patients the regular use of properly applied broad-spectrum sunscreen and the use of sun-protective clothing such as hats, long sleeves, and long pants when outdoors, as well as avoidance of UV rays either from sunlight, or from UV ray-emitting indoor tanning devices.
3) Oncology providers should be vocal supporters of skin cancer prevention policies and should educate patients of color, so that they understand that they are also at risk of skin cancer.
American Society of Clinical Oncology Policy Statement on Skin Cancer Prevention. Alberg AJ, LoConte NK, Foxhall L, et al. JCO Oncology Practice. 2020;16:490-499.
Five Year Analysis of Adjuvant TAFINLAR® plus MEKINIST® in Stage III Melanoma
SUMMARY: It is estimated that in the US, approximately 100,350 new cases of melanoma will be diagnosed in 2020 and approximately 6,850 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma, with a 5-year survival rate of 98% for Stage I disease and 90% for Stage II disease. Stage III malignant melanoma is a heterogeneous disease and the risk of recurrence is dependent on the number of positive nodes as well as presence of palpable versus microscopic nodal disease. Further, patients with a metastatic focus of more than 1 mm in greatest dimension in the affected lymph node, have a significantly higher risk of recurrence or death, than those with a metastasis of 1 mm or less. Patients with Stage IIIA disease have a disease-specific survival rate of 78%, whereas those with Stage IIIB and Stage IIIC disease have disease-specific survival rates of 59% and 40% respectively.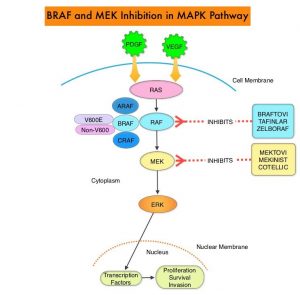
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas and result in constitutive activation of the MAPK pathway.
TAFINLAR® (Dabrafenib) is a selective oral BRAF inhibitor and MEKINIST® (Trametinib) is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway. In patients with BRAF V600 mutation-positive unresectable or metastatic melanoma, a combination of TAFINLAR® and MEKINIST® resulted in a median Overall Survival (OS) of more than 2 years, with approximately 20% of the patients remaining progression free at 3 years. These encouraging results led to the study of this combination in patients with Stage III melanoma, with BRAF V600E or V600K mutations, after complete surgical resection.
COMBI-AD, an international, multi-center, randomized, double-blind, placebo-controlled, Phase III trial, in which 870 patients with completely resected, Stage III melanoma and with BRAF V600E or V600K mutations were enrolled. Patients were randomly assigned in a 1:1 to receive TAFINLAR® 150 mg orally twice daily in combination with MEKINIST® 2 mg orally once daily (N=438) or two matched placebos (N=432). Treatment was given for 12 months. Eligible patients had undergone completion lymphadenectomy, with no clinical or radiographic evidence of residual regional node disease. None of the patients had received previous systemic anticancer treatment or radiotherapy for melanoma. BRAF V600 mutation status was confirmed in primary tumor tissue or lymph node tissue by a central reference laboratory. The median age was 50 years. Both treatment groups were well balanced and 18% had Stage IIIA disease, 41% had Stage IIIB disease, and 40% had Stage IIIC disease. Of the enrolled patients, 91% had a BRAF V600E mutation, and 9% had a BRAF V600K mutation. The Primary end point was Relapse Free Survival (RFS) and Secondary end points included Overall Survival (OS), Distant metastasis-free survival, Freedom from relapse, and Safety.
The authors had previously reported that at a median follow up of 2.8 years, the estimated 3-year RFS rate was 58% in the combination therapy group and 39% in the placebo group (HR=0.47; P<0.001), and this represented a 53% lower risk of relapse. At the time of this analysis, median RFS rate had not been reached in the combination therapy group, and was 16.6 months in the placebo group. The improved RFS benefit with the combination therapy was consistent across patient subgroups, regardless of lymph node involvement or primary tumor ulceration. The risk of distant metastases or death was reduced by 49% with the combination therapy versus placebo (HR=0.51; P<0.001).
The authors in this publication reported the results for RFS and Distant metastasis-free survival at 5 years. Overall survival was not analyzed as the data was not mature. The minimum duration of follow up was 59 months. The RFS at 5 years 52% with TAFINLAR® plus MEKINIST® and 36% with placebo (HR for relapse or death=0.51). The Distant metastasis-free survival at 5 years was 65% with TAFINLAR® plus MEKINIST® and 54% with placebo (HR for distant metastasis or death=0.55). As has been reported in previous studies, majority of relapses occurred, within the first 3 years after surgery. There were no clinically meaningful differences noted in the incidence or severity of serious Adverse Events during the follow up period.
It was concluded that in this 5-year analysis of extended follow up from the COMBI-AD trial, 12 months of adjuvant therapy with a combination of TAFINLAR® and MEKINIST® resulted in longer Relapse Free and Distant metastasis-free Survival, compared to placebo, in patients with resected Stage III melanoma with BRAF V600 mutations.
Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. Dummer R, Hauschild A, Santinami M, et al. N Engl J Med 2020; 383:1139-1148
Association Between Immune Related Adverse Events and Recurrence Free Survival in Stage III Melanoma
SUMMARY: It is estimated that in the US, approximately 100,350 new cases of melanoma will be diagnosed in 2020 and approximately 6,850 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Stage III malignant melanoma is a heterogeneous disease and the risk of recurrence is dependent on the number of positive nodes as well as presence of palpable versus microscopic nodal disease. Further, patients with a metastatic focus of more than 1 mm in greatest dimension in the affected lymph node, have a significantly higher risk of recurrence or death, than those with a metastasis of 1 mm or less. Patients with Stage IIIA disease have a disease-specific survival rate of 78%, whereas those with Stage IIIB and Stage IIIC disease have disease-specific survival rates of 59% and 40% respectively.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions by switching off the immune system T cells. Immune checkpoint proteins/receptors include CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152) and PD-1(Programmed cell Death 1). Checkpoint inhibitors unleash the T cells resulting in T cell proliferation, activation, and a therapeutic response. Immunotherapy with Immune Checkpoint Inhibitors (ICIs) has revolutionized cancer care and has become one of the most effective treatment options by improving Overall Response Rate and prolongation of survival across multiple tumor types.
Immune-related Adverse Events (irAEs) are commonly observed following treatment with ICIs. An association between irAEs and improved outcomes has been reported, among patients with malignant melanoma and lung cancer, treated with ICIs such as anti-CTLA-4 and anti-PD-1 antibodies. It however remains unclear whether immune-related Adverse Events (irAEs) indicate drug activity in patients treated with ICIs.
The European Organization for Research and Treatment of Cancer (EORTC) 1325/(KEYNOTE-054) trial is a randomized, double-blind, placebo-controlled Phase III study which enrolled 1019 patients with completely resected, Stage IIIA, IIIB or IIIC Melanoma. Patients were randomly assigned 1:1 to receive KEYTRUDA® 200 mg IV every three weeks (N=514) or placebo (N=505) as adjuvant therapy, for a total of 18 doses (approximately 1 year) or until disease recurrence or unacceptable toxicity. KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1 monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response, and unleashing the tumor-specific effector T cells.
This study met the Primary end point of Recurrence-Free Survival (RFS), in this high-risk Stage III melanoma patients. KEYTRUDA® was associated with significantly longer Recurrence-Free Survival (RFS) compared to placebo in the overall intent-to-treat population, with a 1-year RFS rate of 75.4% versus 61.0% respectively (HR for recurrence or death=0.57; P<0.001). This suggested that the risk of recurrence or death in the total population was 43% lower in the KEYTRUDA® group than in the placebo group.
The authors in this publication investigated the association between immune-related Adverse Events (irAEs) and Recurrence-Free Survival (RFS) in the KEYNOTE-054 clinical trial, adjusting for age, sex and stage of the disease and also investigated the influence of systemic steroid use on outcome. Of 1011 patients who received treatment with KEYTRUDA® therapy or placebo, 61.5% were men and 38.5% were women. About 25% were 65 years and older and 37% of patients were younger than 50 years. The onset of the first irAE occurred within the first 6 months of treatment for majority of the patients who experienced an irAE and the common irAEs included endocrine disorders such as hypothyroidism or hyperthyroidism, and vitiligo. The incidence of irAEs was 37.4% in the KEYTRUDA® group and 9% in the placebo group, and in each treatment group, the incidence of irAEs was similar for men and women, for younger and older patients, and across different disease stages.
Consistent with previously published results in the intent-to-treat population, a prolonged RFS was observed in the KEYTRUDA® group compared with the placebo group, among patients who started the treatment allocated at the time of randomization (HR=0.56). The occurrence of an irAE was associated with a longer RFS in the KEYTRUDA® group (HR=0.61; P=0.03), but not in the placebo group (HR=1.37; P=0.21). Compared with the placebo arm, the reduction in the hazard of recurrence or death was substantially higher (P=0.03) after the onset of an irAE (HR=0.37), than without or before the onset of an irAE (HR=0.62), in patients who started KEYTRUDA® treatment. Similar results were obtained in each sex group and when only endocrine AEs were considered. Steroid are known to be immune-suppressive and treatment with KEYTRUDA® was less effective when steroids were used after the onset of an irAE.
It was concluded from this secondary analysis that the occurrence of an irAE was associated with a longer Relapse-Free Survival, among patients treated with KEYTRUDA®.
Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo. A Secondary Analysis of a Randomized Clinical Trial. Eggermont AM, Kicinski M, Blank CU, et al. JAMA Oncol. 2020;6:519-527.
FDA Approves IO in Combination with Targeted Therapies for BRAF Positive Advanced Melanoma
SUMMARY: The FDA on July 30, 2020, approved TECENTRIQ® (Atezolizumab), in combination with COTELLIC® (Cobimetinib) and ZELBORAF® (Vemurafenib), for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma. It is estimated that in the US, approximately 100,350 new cases of melanoma will be diagnosed in 2020 and approximately 6,850 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma, with a 5-year survival rate of 98% for Stage I disease and 90% for Stage II disease. Patients with locally advanced or metastatic melanoma historically have had poor outcomes. With the development and availability of immune checkpoint inhibitors and BRAF and MEK inhibitors, this patient group now has significantly improved outcomes.
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been detected in 6-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas, and result in constitutive activation of the MAPK pathway.
ZELBORAF® (Vemurafenib), a selective oral inhibitor of mutated BRAF, demonstrated significant improvement in Progression Free Survival (PFS) and Overall Survival (OS), compared to Dacarbazine. Squamous cell carcinomas were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. MEK gene is downstream from RAF in the MAPK pathway. The addition of a selective inhibitor of MEK gene such as COTELLIC® (Cobimetinib) to a BRAF inhibitor such as ZELBORAF® has addressed some of these limitations, in previously published studies, with improvement in Objective Response Rates (ORR) and decrease in the incidence of cutaneous secondary cancers. coBRIM is a multicenter, randomized, Phase III study in which the efficacy and safety of COTELLIC® combined with ZELBORAF®, was evaluated in previously untreated patients, with advanced BRAF-mutated melanoma. The final analysis of this trial evaluated the 5-year survival data, and the OS was over 30% in patients who received the combination therapy, with a Complete Response (CR) rate was about 20%.
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody, designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, thereby blocking its interactions with PD-1 and B7.1 receptors. PD-L1 inhibition may prevent T-cell deactivation and further enable the activation of T cells. The 5 year OS among patients receiving PD1 targeted immunotherapy is about 34%, with a median OS of 17-20 months. With the approval of multiple therapeutic options for the management of patients with BRAF-mutant melanoma, treatment decisions have become increasingly complex. In patients with limited disease burden, immunotherapy with checkpoint inhibitors is favored by most clinicians, based on the long term data supporting the durability of responses with immunotherapies, but response rates are lower. On the contrary, BRAF-targeted agents are utilized in patients with extensive, symptomatic disease and active brain metastases, as the response rates are higher but are short lived. The optimal sequence of these therapeutic strategies in order to improve long-term patient outcome, has remained unclear.
Preclinical studies suggested that combining these two targeted therapies with a checkpoint inhibitor might overcome the limitations of each class and potentially lead to more durable responses. The safety and efficacy of combining TECENTRIQ® with COTELLIC® (MEK inhibitor) and ZELBORAF® (BRAF inhibitor), in patients with BRAFV600-mutated metastatic melanoma, was evaluated in a Phase I study, with promising results, and a 28-day run-in period with COTELLIC® and ZELBORAF® was associated with an increase in proliferating CD4+ T-helper cells, without increasing the T-regulatory cells (Tregs). Tumor cells use Tregs as a shield to protect themselves against anti-tumor immune response and Tregs remain a hurdle in achieving the complete potential of anti-cancer therapies including immunotherapy. The aim of IMspire 150 trial was to determine if combining checkpoint inhibitor with two targeted therapies would improve efficacy.
IMspire150 is a pivotal, placebo-controlled, international, multicenter, double-blinded, Phase III trial, in which 514 treatment-naive patients with Stage IIIc and Stage IV, BRAF V600–mutant malignant melanoma were enrolled. Patients were randomly assigned 1:1 to treatment with the doublet combination or the triplet therapy. Doublet therapy given to the control group of patients consisted of ZELBORAF® 960 mg orally twice daily plus COTELLIC® at 60 mg orally, on days 1 to 21 of a 28 day cycle. In the experimental or triplet therapy group, there was a 28-day run-in with ZELBORAF® plus COTELLIC® alone, dosed similar to the control group (cycle 1), following which patients received TECENTRIQ® 840 mg IV on Days 1 and 15 of each 28 day cycle starting cycle 2, in combination with ZELBORAF® at a lower dose of 720 mg orally twice daily and COTELLIC® 60 mg orally once daily. Treatment was continued until disease progression, or unacceptable toxicity. Both treatment groups were well balanced, median patient age was 54 years, 58% were male and 94% of patients had Stage IV disease. The Primary endpoint was investigator-assessed Progression Free Survival (PFS). Secondary end points included Objective Response Rates (ORR), Duration of Response (DOR), and Overall Survival (OS).
The combination of immunotherapy with targeted therapies was significantly superior to targeted therapies alone. At a median follow up of 18.9 months, the median PFS with the triplet combination was 15.1 months versus 10.6 months with the doublet therapy (HR=0.78; P=0.025). This represented a 22% reduction in the risk of disease progression. This benefit was observed across all subgroups including age, disease burden, LDH level, and extent of tumor involvement by organ site. Although Objective Response Rates were similar in both treatment groups, the median Duration of Response was 21.0 months with triplet combination versus 12.6 months for the doublet therapy. The OS data were not mature at the time of this analysis, but interim analysis however showed a median OS of 28.8 months with the triplet combination versus 25.1 months with doublet therapy. Both treatment groups had comparable toxicities. Among those patients receiving triplet combination, the most common toxicities were rash, fever, fatigue, nausea, pruritus, stomatitis, musculoskeletal pain, hepatotoxicity, edema, hypothyroidism, and photosensitivity.
It was concluded that in treatment-naive patients with advanced BRAF V600-mutant malignant melanoma, TECENTRIQ® in combination with ZELBORAF® and COTELLIC® significantly and clinically improved Progression Free Survival, when compared to placebo in combination with ZELBORAF® and COTELLIC®.
Evaluation of atezolizumab (A), cobimetinib (C), and vemurafenib (V) in previously untreated patients with BRAFV600 mutation-positive advanced melanoma: Primary results from the phase 3 IMspire150 trial. McArthur GA, Stroyakovskiy D, Gogas H, et al. Presented at: the 2020 AACR Annual Virtual Meeting I; April 27-28, 2020. Abstract CT012.
