SUMMARY: The American Cancer Society’s estimates that for 2021, about 106,110 new cases of melanoma will be diagnosed in the United States and 7,180 people are expected to die of the disease. The rates of melanoma have been rising rapidly over the past few decades, but this has varied by age.
A better understanding of Immune checkpoints has opened the doors for the discovery of novel immune targets. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By blocking the Immune checkpoint proteins, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.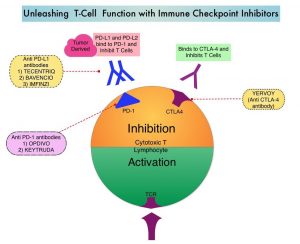
YERVOY® (Ipilimumab) is a fully human immunoglobulin G1 monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA-4, and was the first systemic therapy in randomized Phase III trials, to show prolonged Overall Survival (OS) in patients with advanced melanoma. YERVOY® in a pooled analysis of data from 12 studies showed a 3-year Overall Survival of 26% among treatment naive patients, and survival up to 10 years in approximately 20% of all patients, with advanced melanoma. The two PD-1 inhibitors of interest are OPDIVO® (Nivolumab) and KEYTRUDA® (Pembrolizumab), which are fully human, Immunoglobulin G4, anti-PD-1 targeted monoclonal antibodies that bind to the PD-1 receptor, and block its interaction with ligands PD-L1 and PD-L2, following which the tumor-specific effector T cells are unleashed. They are thus able to undo PD-1 pathway-mediated inhibition of the immune response. When compared with YERVOY® in patients with advanced melanoma, PD-1 inhibitors, both OPDIVO® and KEYTRUDA® have demonstrated superior Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR), with a better safety profile. OPDIVO® in combination with YERVOY® in a Phase I study resulted in an Overall Survival of 68% at 3 years among patients with advanced melanoma, regardless of prior therapies.
CheckMate 067 is a double-blind Phase III study in which patients with previously untreated advanced melanoma were randomly assigned in a 1:1:1 ratio to receive one of the three regimens: OPDIVO® 1 mg/kg every 3 weeks plus YERVOY® 3 mg/kg every 3 weeks for four doses, followed by OPDIVO® 3 mg/kg every 2 weeks (N=314); OPDIVO® 3 mg/kg every 2 weeks plus placebo (N=316); or YERVOY® 3 mg/kg every 3 weeks for four doses plus placebo (N=315). Randomization was stratified according to BRAF mutation status, metastasis stage, and Programmed cell Death Ligand 1 (PD-L1) status. Treatment was continued until disease progression or unacceptable toxicities. The two Primary end points were PFS and OS in the OPDIVO® plus YERVOY® group, and in the OPDIVO® group versus the YERVOY® group.
As previously reported, there was a durable and sustained clinical benefit at 5 years, with superior PFS and OS among patients treated with OPDIVO® plus YERVOY® combination therapy or with OPDIVO® alone, compared with single agent YERVOY®. The authors in this publication reported the efficacy and safety outcomes in this untreated, unresectable Stage III or IV patients with advanced melanoma, after an extended follow up of 6.5 years.
The median Overall Survival for patients treated with OPDIVO® plus YERVOY® combination therapy was 72.1 months, for those treated with single agent OPDIVO® was 36.9 months, compared with 19.9 months with single agent YERVOY®. At the time of analysis at 6.5 years, 49% of patients treated with OPDIVO® plus YERVOY® were alive, compared to 42% of those treated with OPDIVO® alone and 23% of those treated with single agent YERVOY®. The PFS at 6.5 years was 34% for the OPDIVO® plus YERVOY® group, 29% for the OPDIVO® alone group, and 7% for the YERVOY® group.
It was concluded that the results from the 6.5 year analysis showed durable improved outcomes with OPDIVO® plus YERVOY®, and OPDIVO® alone, when compared to single agent YERVOY®, among patients with advanced melanoma. Further, there was an improvement in OS and PFS with OPDIVO® plus YERVOY®, over OPDIVO® alone. The authors added that this analysis represents the longest follow up from a Phase III melanoma trial in the modern checkpoint inhibitor combination therapy and targeted therapy era.
CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. J Clin Oncol 39, 2021 (suppl 15; abstr 9506)

