Dual Inhibition Improves Outcomes for Patients with BRAF-Mutated Colorectal Tumors
SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 135,430 new cases of ColoRectal Cancer will be diagnosed in the United States in 2017 and over 50,260 patients are expected to die of the disease. The DNA MisMatchRepair (MMR) system is responsible for molecular surveillance and works as an editing tool that identifies errors within the microsatellite regions of DNA and removes them. Defective MMR system leads to MSI (Micro Satellite Instability) and hypermutation, triggering an enhanced antitumor immune response. MSI (Micro Satellite Instability) is therefore a hallmark of defective/deficient DNA MisMatchRepair (MMR) system and occurs in 15% of all colorectal cancers. Defective MisMatchRepair can be a sporadic or heritable event. Approximately 65% of the MSI tumors are sporadic and MSI-High tumors tend to have better outcomes. Patients with stage IV colorectal cancer are now routinely analyzed for extended RAS and BRAF mutations. KRAS mutations are predictive of resistance to Epidermal Growth Factor Receptor (EGFR) targeted therapy. Approximately 5-10% of all metastatic CRC tumors present with BRAF V600 mutations and BRAF V600 is recognized as a marker of poor prognosis in this patient group. These patients tend to have aggressive disease with a higher rate of peritoneal metastasis and do not respond well to standard treatment intervention. Approximately 25% of the BRAF-mutated population in the metastatic setting has MSI-High tumors, but MSI-High status does not confer protection to this patient group.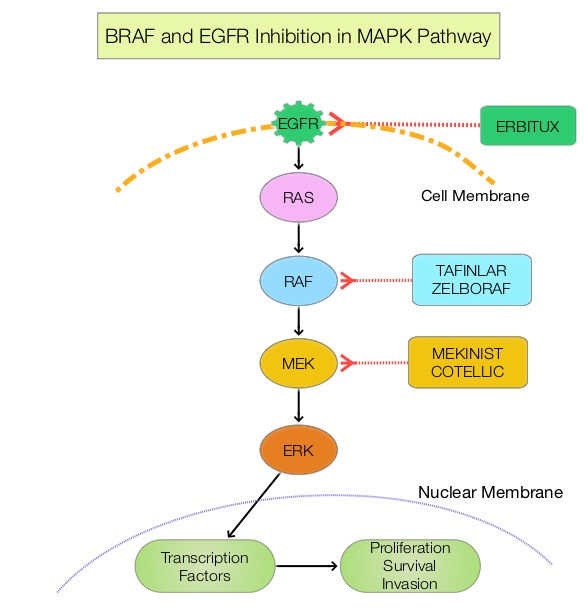
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules.BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. The BRAF V600 mutations results in constitutive activation of the MAP kinase pathway. Inhibiting BRAF can transiently reduce MAP kinase signaling. However, this can result in feedback upregulation of EGFR signaling pathway, which can then reactivate the MAP kinase pathway. This aberrant signaling can be blocked by dual inhibition of both BRAF and EGFR.
ZELBORAF® (Vemurafenib), is a selective oral inhibitor of mutated BRAF whereas ERBITUX® (Cetuximab) is a monoclonal antibody targeting Epidermal Growth Factor Receptor (EGFR). Preclinical studies have shown that adding CAMPTOSAR® (Irinotecan) to ZELBORAF® and ERBITUX®, in patients with refractory BRAF V600E metastatic CRC, led to a durable responses and this combination was safe and tolerable. However, both single agent ZELBORAF® and ERBITUX® were shown to have limited activity in this patient group.
Based on this scientific rationale, a phase II trial was conducted (SWOG 1406), in which 106 metastatic ColoRectal Cancer patients, with mutations in BRAF V600 and extended RAS wild-type, were enrolled. Patients were randomized to receive CAMPTOSAR® 180 mg/m2 IV every 14 days and ERBITUX® 500 mg/m2 IV every 14 days, with or without ZELBORAF® 960 mg orally twice daily. The median age was 62 years and about 50% of patients had received 1 prior regimen for metastatic or locally advanced unresectable metastatic CRC, and 39% had received prior treatment with CAMPTOSAR® . Prior therapy with anti-EGFR agent or RAF or MEK inhibitors was not allowed. Crossover from the control arm to the experimental group was allowed, after documented disease progression. The primary endpoint was Progression Free Survival.
The median Progression Free Survival was 4.4 months with the triplet, versus 2.0 months with CAMPTOSAR® plus ERBITUX® (HR=0.42; P =0.0002). The response rate was 16% versus 4%, and the Disease Control Rate was 67% versus 22% (P =0.001), with a higher Duration of Response with the addition of ZELBORAF® to CAMPTOSAR® and ERBITUX® (Triplet). Approximately 50% of the patients in the control group crossed over to the experimental group at the time of disease progression. Overall Survival data and efficacy at cross-over, data, remain immature. Patients in the experimental group (Triplet group) experienced more grade 3/4 toxicities such as neutropenia, anemia and nausea, and this increase was attributed to increased duration of exposure to therapy.
The authors concluded that the addition of ZELBORAF® to the combination of CAMPTOSAR® and ERBITUX® resulted in a 58% reduction in the risk of disease progression and a higher Disease Control Rate, suggesting that simultaneous EGFR and BRAF inhibition (Dual Inhibition) is effective in BRAF V600 mutated ColoRectal Cancer. Subgroup analysis will examine the role of CAMPTOSAR® pre-treatment and the outcomes of patients based on tumor MicroSatellite Instability. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). Kopetz S, McDonough SL, Morris VK, et al. J Clin Oncol 35, 2017 (suppl 4S; abstract 520)

