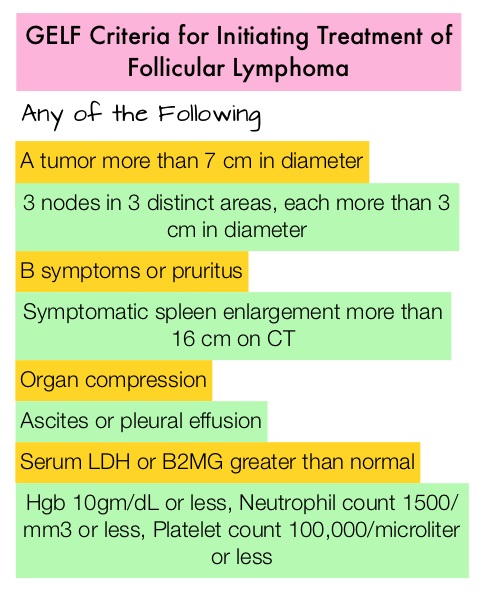
SUMMARY: The FDA on February 26, 2016, approved GAZYVA® (Obinutuzumab) for use in combination with TREANDA® (Bendamustine) followed by GAZYVA® monotherapy for the treatment of patients with Follicular Lymphoma (FL) who relapsed after, or are refractory to, a RITUXAN® (Rituximab) containing regimen. GAZYVA® was previously approved for use in combination with Chlorambucil for the treatment of patients with previously untreated Chronic Lymphocytic Leukemia. The American Cancer Society estimates that in 2016, about 72,580 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,150 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT) lymphoma, Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas. Advanced stage indolent NHL are not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining quality of life (QoL), has been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach, whereas those with B symptoms (fever, night sweats, and weight loss), painful lymphadenopathy/splenomegaly, organ compromise and cytopenias are generally considered candidates for therapy.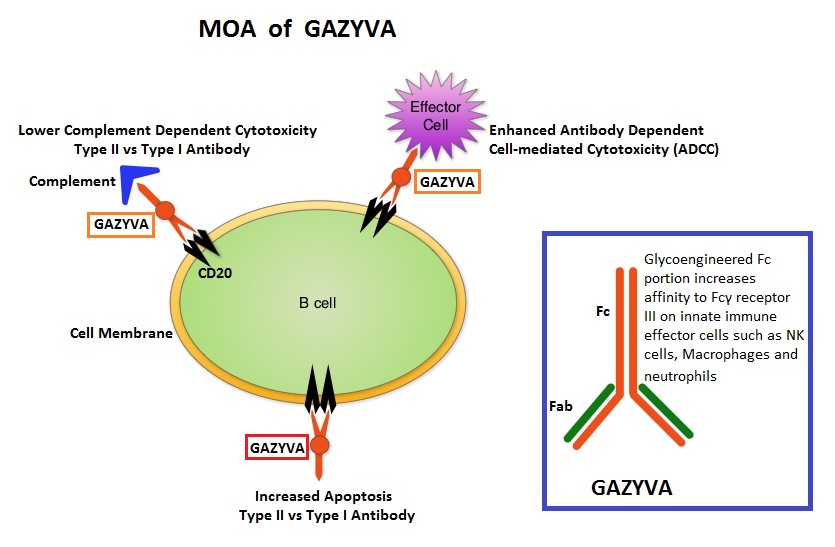
GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis is significantly enhanced, whereas it induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.
GADOLIN is a pivotal multicenter, open-label phase III, study in which TREANDA® (Bendamustine) alone was compared with TREANDA® plus GAZYVA® followed by GAZYVA® maintenance, in patients with indolent NHL (iNHL), refractory to RITUXAN®. Four hundred and thirteen (N=413) RITUXAN® refractory iNHL were randomized and patients in the control arm received TREANDA® 120 mg/m2 IV on days 1 and 2 every 28 days for a total of 6 cycles. Patients in the experimental arm received TREANDA® 90 mg/m2 IV on days 1 and 2 every 28 days for 6 cycles and GAZYVA® 1000mg IV days 1,8 and 15 every 28 days of cycle 1 and on day 1 of cycles 2-6. In patients with non-progressive disease in the experimental arm, GAZYVA® was continued (maintenance) every 2 months for up to 2 years. Both treatment groups were well balanced and the median age was 63 years, with a median of two prior lines of therapy. More than 90% of patients in each treatment group were refractory to their previous therapy and between 76% and 81% were double-refractory to both RITUXAN® and an alkylating agent. The Primary end point was Progression Free Survival (PFS) and Secondary end points included Overall Survival and Response Rate.
The study was unblinded at the time of planned interim analysis and had to be halted early, upon recommendations from the Independent Data Monitoring Committee, as the primary end point was reached. The median Progression Free Survival was 29 months with GAZYVA®/ TREANDA® plus maintenance GAZYVA® versus 14 months with TREANDA® monotherapy and no maintenance (HR=0.52; P<0.001). This meant a 45% reduction in the rate of disease progression. There was however no difference in the Response Rates between the treatment groups and the best Overall Response Rate up to 12 months from start of treatment was, 76.6% in the TREANDA® alone group and 78.6% in the TREANDA® plus GAZYVA® group. Median Overall Survival has not yet been reached in either arm and longer follow up is needed. The combination experimental group experienced more grade 3 adverse events such as infusion related reactions and neutropenia whereas the TREANDA® alone group experienced more thrombocytopenia, anemia and pneumonia.
The authors concluded that GAZYVA® in combination with TREANDA® is superior to TREANDA® alone, in patients with RITUXAN® refractory indolent Non Hodgkin Lymphoma, with a significant improvement in Progression Free survival. The lack of difference in the Response Rate begs the question, if the improvement in PFS was predominantly contributed by the continuous maintenance treatment with GAZYVA®. GADOLIN: Primary results from a phase III study of obinutuzumab plus bendamustine compared with bendamustine alone in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Sehn LH, Chua NS, Mayer J, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA8502)


 The beneficial effects of Vitamin D in malignancies has been attributed to its antiproliferative and antiangiogenic properties, as well as its effects on cell differentiation, promotion of apoptosis and its ability to decreases oxidative DNA damage. Further, macrophages play an important role in the human body’s response to therapy with monoclonal antibodies, an integral part of Follicular Lymphoma therapies and low serum Vitamin D levels may interfere with macrophage function and this may explain poor outcomes in some Follicular Lymphoma patients with low Vitamin D levels
The beneficial effects of Vitamin D in malignancies has been attributed to its antiproliferative and antiangiogenic properties, as well as its effects on cell differentiation, promotion of apoptosis and its ability to decreases oxidative DNA damage. Further, macrophages play an important role in the human body’s response to therapy with monoclonal antibodies, an integral part of Follicular Lymphoma therapies and low serum Vitamin D levels may interfere with macrophage function and this may explain poor outcomes in some Follicular Lymphoma patients with low Vitamin D levels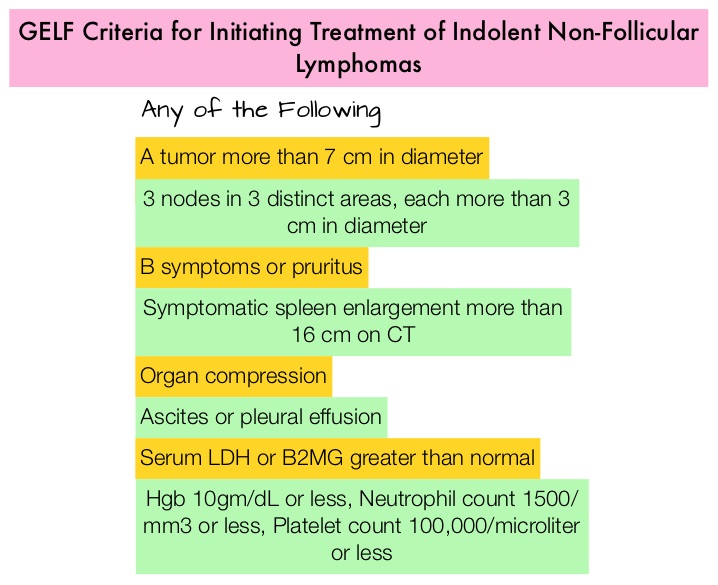
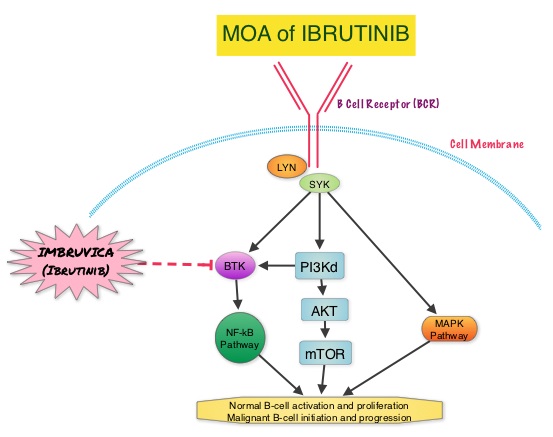 About 90% of patients with Waldenstrom Macroglobulinemia demonstrate a mutation in chromosome 3p (MYD88 L265P), which is specific to WM and may be an early oncogenic event in WM pathogenesis.. It appears that MYD88 L265P promotes malignant cell proliferation via the Bruton’s Tyrosine Kinase (BTK) signaling pathway. Mutations in CXCR4 gene are present in 30% of patients with WM, and their expression induces BTK activity and may confer resistance to BTK inhibitors. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). Preliminary studies in WM patients have revealed that IMBRUVICA® prevents the binding of MYD88 L265P (mutated gene) to BTK thereby selectively killing tumor cells. On the other hand it was noted that a major response to IMBRUVICA® was less likely when mutations in CXCR4 gene were present in the tumor cells. With this molecular understanding of WM, the authors enrolled 63 patients with relapsed/refractory symptomatic WM and were treated with IMBRUVICA®, 420 mg PO daily for 2 years or until disease progression or unacceptable toxicity. Anemia was main indication for treatment initiation (87.3% of patients) and the mean baseline hemoglobin level was 10.5 g/dL, mean serum IgM level was 3610 mg/dL, 70% had bone marrow involvement and 60% of patients had lymphadenopathy.
About 90% of patients with Waldenstrom Macroglobulinemia demonstrate a mutation in chromosome 3p (MYD88 L265P), which is specific to WM and may be an early oncogenic event in WM pathogenesis.. It appears that MYD88 L265P promotes malignant cell proliferation via the Bruton’s Tyrosine Kinase (BTK) signaling pathway. Mutations in CXCR4 gene are present in 30% of patients with WM, and their expression induces BTK activity and may confer resistance to BTK inhibitors. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). Preliminary studies in WM patients have revealed that IMBRUVICA® prevents the binding of MYD88 L265P (mutated gene) to BTK thereby selectively killing tumor cells. On the other hand it was noted that a major response to IMBRUVICA® was less likely when mutations in CXCR4 gene were present in the tumor cells. With this molecular understanding of WM, the authors enrolled 63 patients with relapsed/refractory symptomatic WM and were treated with IMBRUVICA®, 420 mg PO daily for 2 years or until disease progression or unacceptable toxicity. Anemia was main indication for treatment initiation (87.3% of patients) and the mean baseline hemoglobin level was 10.5 g/dL, mean serum IgM level was 3610 mg/dL, 70% had bone marrow involvement and 60% of patients had lymphadenopathy. 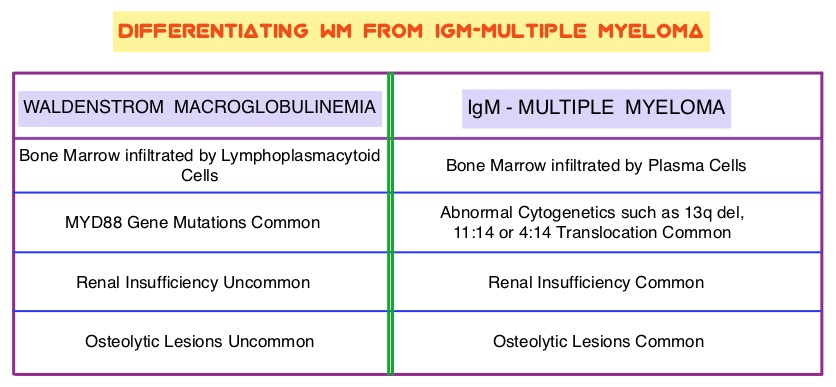 Sanger sequencing was used to determine MYD88 and CXCR4 mutations in the bone marrow lymphoplasmacytic cells. At best response, the median serum IgM levels declined to 1340 mg/dL (P<0.00001), median hemoglobin rose to 12.6 g/dL, (P<0.00001). At 6 months, bone marrow assessment post treatment, demonstrated a reduction in WM disease involvement from 70% to 45% (P=0.0006). With a median follow up at 6 cycles, the best overall response rate was 81% and a median time to response was 4 weeks. In patients who underwent tumor sequencing, mutations in CXCR4 gene impacted response rates. The major response rate for patients with wild-type CXCR4 gene was 77% compares to 30% in those with CXCR4 mutations (p=0.018). Further, patients with wild-type CXCR4 also had increased peripheral lymphocytosis following treatment with IMBRUVICA® compared to those with CXCR4 mutations (P=0.001). The most common more than grade 2 treatment related toxicities included thrombocytopenia (14.3%) and neutropenia (19.1%). The authors concluded that IMBRUVICA® is highly active in patients with relapsed or refractory Waldenstrom Macroglobulinemia, with rapid reductions in serum IgM level and improved hemoglobin levels. The presence of CXCR4 mutations negatively impact response rates in this patient group. Treon SP, Tripsas CK, Yang G, et al. Presented at: 55th ASH Annual Meeting; December 7-10, 2013; New Orleans, LA. Abstract 251.
Sanger sequencing was used to determine MYD88 and CXCR4 mutations in the bone marrow lymphoplasmacytic cells. At best response, the median serum IgM levels declined to 1340 mg/dL (P<0.00001), median hemoglobin rose to 12.6 g/dL, (P<0.00001). At 6 months, bone marrow assessment post treatment, demonstrated a reduction in WM disease involvement from 70% to 45% (P=0.0006). With a median follow up at 6 cycles, the best overall response rate was 81% and a median time to response was 4 weeks. In patients who underwent tumor sequencing, mutations in CXCR4 gene impacted response rates. The major response rate for patients with wild-type CXCR4 gene was 77% compares to 30% in those with CXCR4 mutations (p=0.018). Further, patients with wild-type CXCR4 also had increased peripheral lymphocytosis following treatment with IMBRUVICA® compared to those with CXCR4 mutations (P=0.001). The most common more than grade 2 treatment related toxicities included thrombocytopenia (14.3%) and neutropenia (19.1%). The authors concluded that IMBRUVICA® is highly active in patients with relapsed or refractory Waldenstrom Macroglobulinemia, with rapid reductions in serum IgM level and improved hemoglobin levels. The presence of CXCR4 mutations negatively impact response rates in this patient group. Treon SP, Tripsas CK, Yang G, et al. Presented at: 55th ASH Annual Meeting; December 7-10, 2013; New Orleans, LA. Abstract 251.
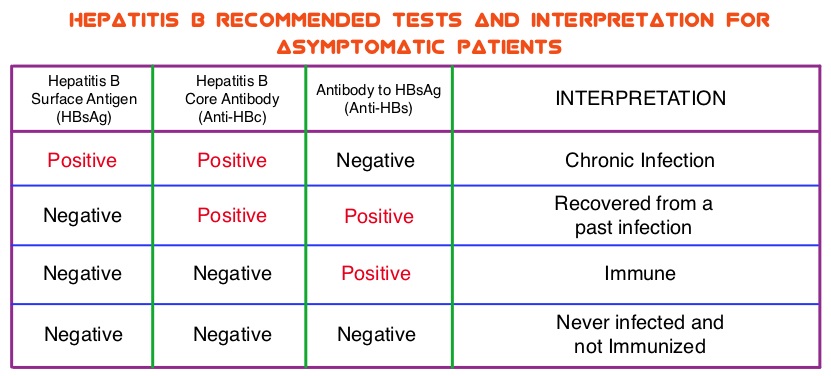 The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients, but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as RITUXAN® (Rituximab). According to the International recommendations, HBV reactivation is defined as the detection of serum HBV DNA of 10 IU/mL or more, by a real-time polymerase chain reaction–based assay. Because of the ambiguity regarding HBV reactivation in lymphoma patients receiving immunosuppressive therapy, the authors conducted a prospective trial to determine the frequency and factors predictive of HBV reactivation in HBsAg-negative, anti-HBc–positive patients treated with RITUXAN® based chemotherapy regimens. In this observational study, 260 patients with hematologic malignancies who were HBsAg-negative, anti-HBc–positive, with undetectable serum HBV DNA (< 10 IU/mL) and treated with RITUXAN® containing chemotherapy, were prospectively monitored every 4 weeks for up to 2 years. Patients were started on BARACLUDE® (Entecavir), when HBV reactivation (serum HBV DNA of 10 IU/mL or more), was documented.
The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients, but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as RITUXAN® (Rituximab). According to the International recommendations, HBV reactivation is defined as the detection of serum HBV DNA of 10 IU/mL or more, by a real-time polymerase chain reaction–based assay. Because of the ambiguity regarding HBV reactivation in lymphoma patients receiving immunosuppressive therapy, the authors conducted a prospective trial to determine the frequency and factors predictive of HBV reactivation in HBsAg-negative, anti-HBc–positive patients treated with RITUXAN® based chemotherapy regimens. In this observational study, 260 patients with hematologic malignancies who were HBsAg-negative, anti-HBc–positive, with undetectable serum HBV DNA (< 10 IU/mL) and treated with RITUXAN® containing chemotherapy, were prospectively monitored every 4 weeks for up to 2 years. Patients were started on BARACLUDE® (Entecavir), when HBV reactivation (serum HBV DNA of 10 IU/mL or more), was documented.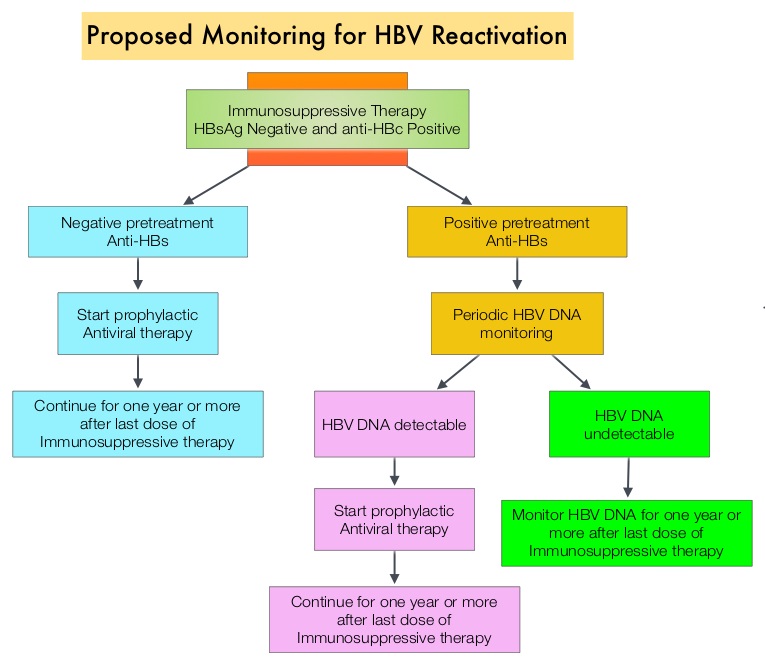 The cumulative rate of HBV reactivation over the 2 year observation period was high at 41.5%. The HBV reactivation occurred at a median of 23 weeks after RITUXAN® treatment and the median HBV DNA level at reactivation was 43 IU/mL. Undetectable antibody level to HBsAg (anti-HBs; < 10 mIU/mL) at baseline, prior to treatment with RITUXAN®, was the only significant risk factor that was strongly associated with HBV reactivation (P=0.009). Patients with negative baseline antibody level to HBsAg (anti-HBs) had a significantly higher 2-year cumulative rate of HBV reactivation, compared with those who had positive baseline antibody level to HBsAg (68.3% vs 34.4%; P=0.012). All patients had normal ALT when HBV reactivation occurred and except for one patient, were HBsAg negative. More importantly, all patients with HBV reactivation were successfully treated with BARACLUDE®. The authors concluded that HBsAg-negative, anti-HBc–positive lymphoma patients, receiving RITUXAN® based chemotherapy regimens experience a high rate of HBV reactivation, with this rate even significantly higher in patients with negative baseline antibody level to HBsAg. Periodic monitoring for HBV reactivation can enable early detection and intervention,thereby avoiding HBV related morbidities and mortality. Seto W, Chan T, Hwang Y, et al. JCO 2014;32:3736-3743
The cumulative rate of HBV reactivation over the 2 year observation period was high at 41.5%. The HBV reactivation occurred at a median of 23 weeks after RITUXAN® treatment and the median HBV DNA level at reactivation was 43 IU/mL. Undetectable antibody level to HBsAg (anti-HBs; < 10 mIU/mL) at baseline, prior to treatment with RITUXAN®, was the only significant risk factor that was strongly associated with HBV reactivation (P=0.009). Patients with negative baseline antibody level to HBsAg (anti-HBs) had a significantly higher 2-year cumulative rate of HBV reactivation, compared with those who had positive baseline antibody level to HBsAg (68.3% vs 34.4%; P=0.012). All patients had normal ALT when HBV reactivation occurred and except for one patient, were HBsAg negative. More importantly, all patients with HBV reactivation were successfully treated with BARACLUDE®. The authors concluded that HBsAg-negative, anti-HBc–positive lymphoma patients, receiving RITUXAN® based chemotherapy regimens experience a high rate of HBV reactivation, with this rate even significantly higher in patients with negative baseline antibody level to HBsAg. Periodic monitoring for HBV reactivation can enable early detection and intervention,thereby avoiding HBV related morbidities and mortality. Seto W, Chan T, Hwang Y, et al. JCO 2014;32:3736-3743
 This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s
This latest approval was based on the results of an international, randomized, open-label phase III trial in which 487 patients with stage II to IV MCL, who were ineligible or not considered for Bone Marrow Transplantation, received VR-CAP (N = 243) or R-CHOP (N = 244). VR- CAP is essentially R-CHOP with the Vincristine replaced by VELCADE®. So, VR-CAP regimen consisted of VELCADE® administered IV at 1.3 mg/m2 on days 1, 4, 8, and 11, RITUXAN® (Rituximab) 375 mg/m2 IV given on day 1, Cyclophosphamide 750 mg/m2 IV on day 1, Doxorubicin 50 mg/m2 IV on day 1 and Prednisone at 100 mg/m2 PO on days 1 to 5 of a 21 day cycle for 6-8 cycles. R-CHOP regimen was exactly similar except that Vincristine 1.4 mg/m2 (max 2 mg) IV was administered on day 1 of each cycle instead of VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Time To Progression (TTP), Time To Next Treatment (TTNT), Overall Survival (OS) and safety. Patients received a median of 6 cycles and after a median follow up of 40 months, patients in the VR-CAP group demonstrated a significantly longer median PFS (25 months vs. 14 months; HR=0.63;P<0.001) with a 37% relative improvement in the PFS compared to those who were treated with standard R-CHOP. Patients in the VR-CAP group also had a higher overall response rate (88 vs 85%) and a higher rate of complete response (44% vs. 34%). The most common adverse reactions occurring in 20% or more of patients receiving the VR-CAP regimen were neutropenia, leukopenia, anemia, thrombocytopenia, lymphopenia, peripheral neuropathy, pyrexia, nausea and diarrhea. Infections were reported for 31% of patients in the VR-CAP group compared to 23% of the patients in the R-CHOP group. The authors concluded that VR-CAP significantly prolonged PFS and consistently improved secondary efficacy endpoints, compared to R-CHOP, in newly diagnosed, Bone Marrow Transplant ineligible Mantle Cell Lymphoma patients with manageable toxicity. Proteosome inhibition with a VELCADE® based chemotherapy regimen has opened the doors for more effective therapies for Mantle Cell Lymphoma patients. Cavalli F, Rooney B, Pei L, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8500)</s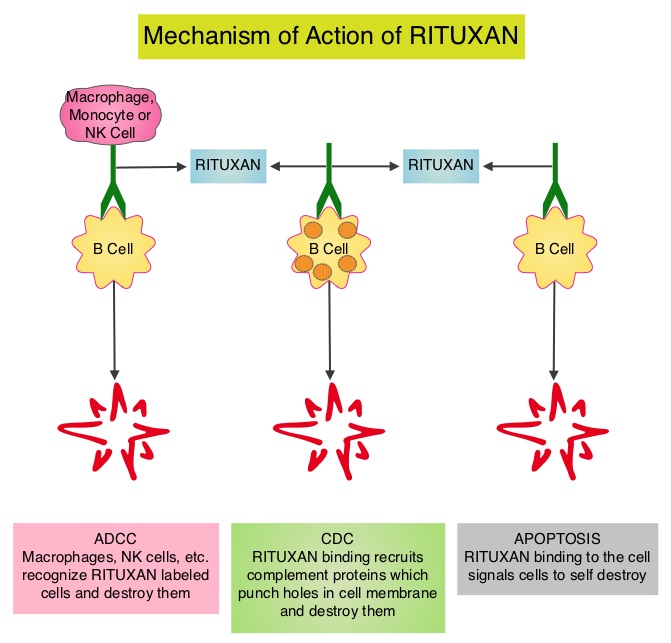 Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102