SUMMARY: The FDA on October 31, 2017, granted accelerated approval to CALQUENCE® (Acalabrutinib) for the treatment of adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. The American Cancer Society estimates that in 2017, about 72,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,140 individuals will die of this disease. In the US, approximately 3,300 new cases of MCL are diagnosed each year. Mantle Cell Lymphoma is an aggressive B-cell lymphoma and accounts for approximately 6% of all Non Hodgkin Lymphomas in adults, and is associated with a high relapse rate, following dose-intensive therapies. Early and late relapses in patients with MCL have been attributed to persistence of residual disease.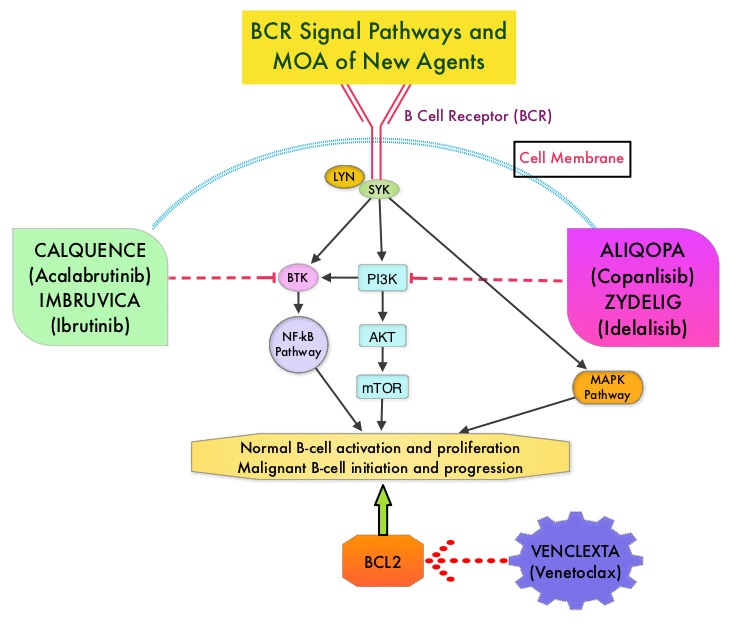
Normal B-cell activation and proliferation is dependent on B-cell receptor (BCR) signaling. This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. Three previously approved agents by the FDA for MCL include, IMBRUVICA® (Ibrutinib), REVLIMID® (Lenalidomide) and VELCADE® (Bortezomib).
CALQUENCE® is a novel, irreversible, second-generation BTK inhibitor, designed to be more potent and selective than IMBRUVICA®. Unlike IMBRUVICA®, CALQUENCE® has reduced off-target activity on EGFR, TEC, etc., which may lead to less untoward toxicities such as bleeding, rash, and atrial fibrillation. The approval of CALQUENCE® was based on ACE-LY-004 study, which is a Phase II, open label, single-arm clinical trial, in which 124 adult patients with Relapsed or Refractory MCL were enrolled. Patients had a confirmed diagnosis of MCL, 93% of the patients had an ECOG PS of 1 or less, median number of prior treatments were 2, which included stem cell transplant for 18% of patients, and 24% of the patients were refractory to their most recent prior treatment. Those treated with a prior BTK inhibitor were excluded from this study. The median age was 68 years. CALQUENCE® was administered orally at 100 mg twice daily until progressive disease or unacceptable toxicity. The Primary endpoint was Objective Response Rate (Complete Response + Partial Response) and Secondary endpoints included Duration of Response (DOR), Progression Free Survival (PFS), Overall Survival (OS) and safety.
At a median follow up of 15.2 months, the Objective Response Rate was 81% with a Complete Response rate of 40% and Partial Response rate of 41%. The median Duration of Response was not yet reached at the time of analysis, with ongoing responses at 20+ months. The response rates were consistent across prespecified subgroups of age, tumor bulk of 10 cm or more and number and types of prior treatment. The median time to best response was 1.9 months. The median Duration of Response (DOR) was not reached and the 12-month DOR was 72%. The median PFS and OS were not reached, whereas the 12-month PFS and OS rates were 67% and 87% respectively. The most common toxicities of any grade included cytopenias, headache, diarrhea, fatigue, myalgia and bruising.
It was concluded that for patients with Relapsed/Refractory Mantle Cell Lymphoma, CALQUENCE® given as a single agent resulted in a high and durable Objective Response Rate as well as Complete Response Rate, with a favorable safety profile. CALQUENCE® is a new treatment option for this aggressive malignancy. Efficacy and Safety of Acalabrutinib Monotherapy in Patients with Relapsed/Refractory Mantle Cell Lymphoma in the Phase 2 ACE-LY-004 Study. Wang M, Rule S, Zinzani PL, et al. 59th Annual Meeting & Exposition Atlanta, GA. December 9-12, 2017. #155

