SUMMARY: The American Cancer Society estimates that in 2017, about 72,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,140 individuals will die of this disease. Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3 to 4% each year. The etiology of Diffuse Large B-Cell Lymphoma is unknown. Contributing risk factors include immunosuppression (AIDS, transplantation setting, autoimmune diseases), ultraviolet radiation, pesticides, hair dyes, and diet. DLBCL is a neoplasm of large B cells and the most common chromosome abnormality involves alterations of the BCL-6 gene at the 3q27 locus, which is critical for germinal center formation. Two major molecular subtypes of DLBCL arising from different genetic mechanisms have been identified, using gene expression profiling: Germinal Center B-cell-like (GCB) and Activated B-Cell-like (ABC). Patients in the GCB subgroup have a higher five year survival rate, independent of clinical IPI risk score whereas patients in the ABC subgroup have a significantly worse outcome. Regardless, R-CHOP regimen (RITUXAN®-Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone), given every 21 days, for 6 cycles, delivered with curative intent, is the current standard of care for patients of all ages, with newly diagnosed DLBCL, regardless of molecular subtype. Approximately 30-40% of patients experience disease progression or relapse, during the first 2 years and attempts to improve on R-CHOP regimen have not been successful. Maintenance treatment strategy following R-CHOP, to better control the disease, delay disease progression and improve long term survival, have included Autologous Stem Cell Transplantation, maintenance treatment with agents such as oral protein kinase inhibitor Enzastaurin and Everolimus. None of these interventions have been successful.
REVLIMID® (Lenalidomide) is an oral immunomodulatory agent (IMiD) with activity in lymphoid malignancies, primarily through immune modulation (repair T-cell immune synapse dysfunction and Natural Killer cell/T-cell effector augmentation). It additionally has antiproliferative effects. REVLIMID® was shown to have significant activity in relapsed DLBCL when given alone or along with RITUXAN®.
The REMARC study is an international, multicenter, double-blind, randomized, placebo-controlled phase III trial which compared REVLIMID® as maintenance therapy with placebo, in elderly patients with DLBCL, who achieved a Complete Response (CR) or Partial Response (PR) to R-CHOP induction treatment. A total of 650 patients who had CR or PR after 6-8 cycles of R-CHOP were randomly assigned in a 1:1 ratio to receive oral REVLIMID® maintenance 25 mg daily or placebo, for 21 days of every 28-day cycle, for 24 months. The median age was 68 years and approximately 90% of the patients had stage III-IV disease. The Primary end point was Progression Free Survival (PFS) and Secondary end points included safety, the percentage of patients who converted from PR to CR, Event Free Survival and Overall Survival (OS).
With a median follow up of 39 months, median PFS was not reached in the REVLIMID® group compared to 58.9 months in the placebo group (HR=0.70; P=0.013) favoring REVLIMID®. This PFS benefit with REVLIMID® maintenance was seen in all predefined subgroups (all age groups, all IPI scores, molecular subtypes, CR versus PR after R-CHOP, Positive versus Negative PET status at the time of randomization). The Overall Survival however was similar between the treatment groups after a longer median follow up of 52 months (P=0.26). The most common grade 3 or 4 toxcities associated with REVLIMID® maintenance were neutropenia and cutaneous reactions.
It was concluded that maintenance treatment with REVLIMID® for 24 months, after obtaining a CR or PR to R-CHOP, significantly prolonged Progression Free Survival in elderly patients with Diffuse Large B-Cell Lymphoma. This is the first randomized study showing a PFS benefit with an immunomodulatory agent as maintenance therapy, in this patient population. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. Thieblemont C, Tilly H, Gomes da Silva M, et al. DOI: 10.1200/JCO.2017.72.6984 Journal of Clinical Oncology – published online before print April 20, 2017

 Hyaluronan is a polysaccharide present in the extracellular matrix of the subcutaneous tissue and is depolymerized by the naturally occurring enzyme Hyaluronidase.
Hyaluronan is a polysaccharide present in the extracellular matrix of the subcutaneous tissue and is depolymerized by the naturally occurring enzyme Hyaluronidase. 
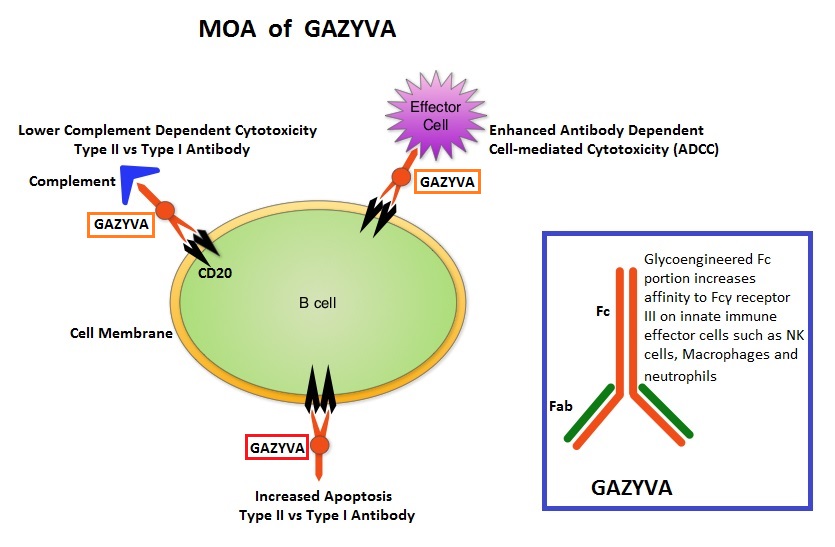
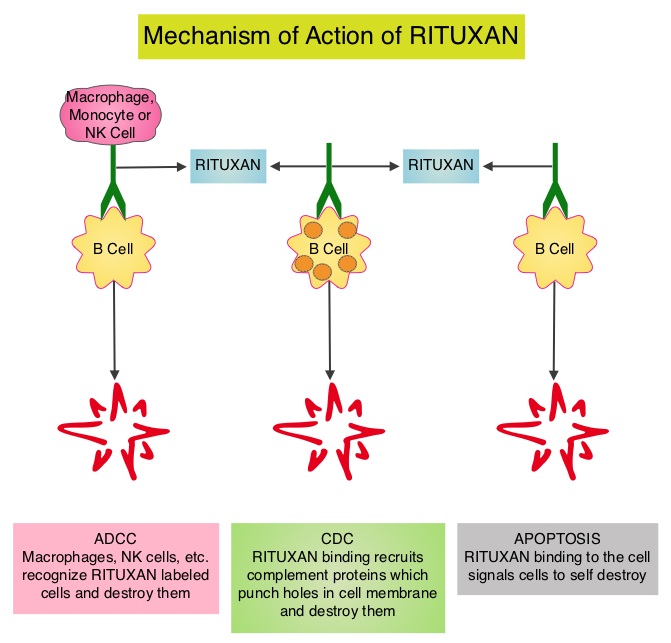
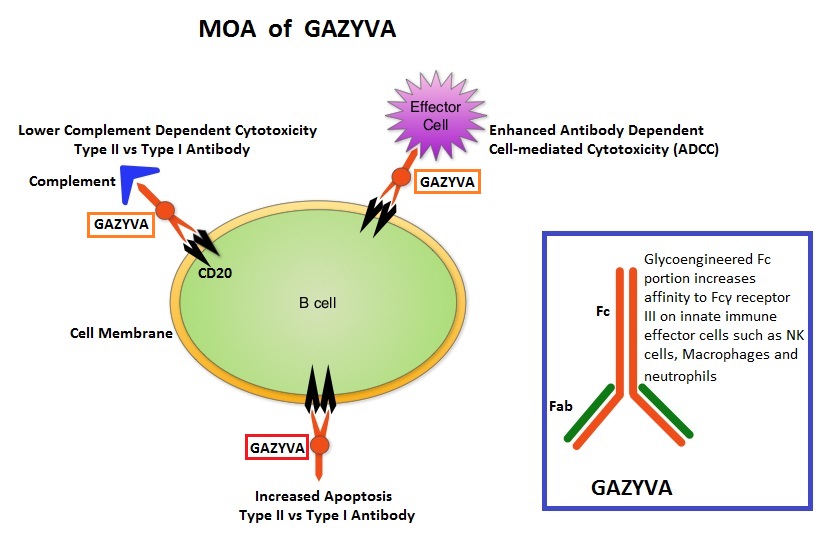 GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis is significantly enhanced, whereas it induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.
GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis is significantly enhanced, whereas it induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.