The FDA on November 16, 2017, granted regular approval to GAZYVA® in combination with chemotherapy, followed by GAZYVA® monotherapy, in patients achieving at least a Partial Remission, for the treatment of adult patients with previously untreated Stage II bulky, III, or IV Follicular Lymphoma (FL). GAZYVA® is a product of Genentech, Inc.
Tag: Non-Hodgkin Lymphoma
ADCETRIS® (Brentuximab vedotin)
The FDA on November 9, 2017 granted regular approval to ADCETRIS®, for the treatment of adult patients with primary cutaneous Anaplastic Large Cell Lymphoma (pcALCL) or CD30-expressing Mycosis Fungoides (MF), who have received prior systemic therapy. ADCETRIS® is a product of Seattle Genetics, Inc.
FDA Grants Approval to CALQUENCE® for Mantle Cell Lymphoma
SUMMARY: The FDA on October 31, 2017, granted accelerated approval to CALQUENCE® (Acalabrutinib) for the treatment of adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. The American Cancer Society estimates that in 2017, about 72,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,140 individuals will die of this disease. In the US, approximately 3,300 new cases of MCL are diagnosed each year. Mantle Cell Lymphoma is an aggressive B-cell lymphoma and accounts for approximately 6% of all Non Hodgkin Lymphomas in adults, and is associated with a high relapse rate, following dose-intensive therapies. Early and late relapses in patients with MCL have been attributed to persistence of residual disease.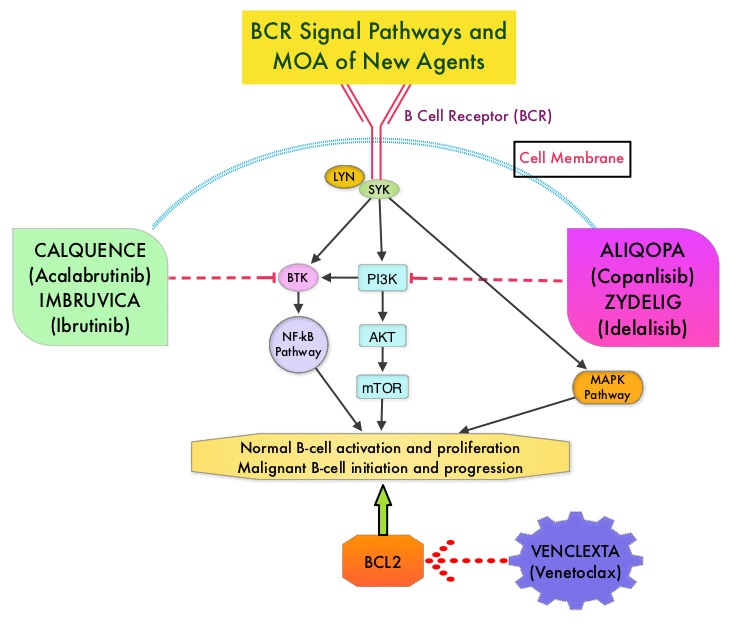
Normal B-cell activation and proliferation is dependent on B-cell receptor (BCR) signaling. This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. Three previously approved agents by the FDA for MCL include, IMBRUVICA® (Ibrutinib), REVLIMID® (Lenalidomide) and VELCADE® (Bortezomib).
CALQUENCE® is a novel, irreversible, second-generation BTK inhibitor, designed to be more potent and selective than IMBRUVICA®. Unlike IMBRUVICA®, CALQUENCE® has reduced off-target activity on EGFR, TEC, etc., which may lead to less untoward toxicities such as bleeding, rash, and atrial fibrillation. The approval of CALQUENCE® was based on ACE-LY-004 study, which is a Phase II, open label, single-arm clinical trial, in which 124 adult patients with Relapsed or Refractory MCL were enrolled. Patients had a confirmed diagnosis of MCL, 93% of the patients had an ECOG PS of 1 or less, median number of prior treatments were 2, which included stem cell transplant for 18% of patients, and 24% of the patients were refractory to their most recent prior treatment. Those treated with a prior BTK inhibitor were excluded from this study. The median age was 68 years. CALQUENCE® was administered orally at 100 mg twice daily until progressive disease or unacceptable toxicity. The Primary endpoint was Objective Response Rate (Complete Response + Partial Response) and Secondary endpoints included Duration of Response (DOR), Progression Free Survival (PFS), Overall Survival (OS) and safety.
At a median follow up of 15.2 months, the Objective Response Rate was 81% with a Complete Response rate of 40% and Partial Response rate of 41%. The median Duration of Response was not yet reached at the time of analysis, with ongoing responses at 20+ months. The response rates were consistent across prespecified subgroups of age, tumor bulk of 10 cm or more and number and types of prior treatment. The median time to best response was 1.9 months. The median Duration of Response (DOR) was not reached and the 12-month DOR was 72%. The median PFS and OS were not reached, whereas the 12-month PFS and OS rates were 67% and 87% respectively. The most common toxicities of any grade included cytopenias, headache, diarrhea, fatigue, myalgia and bruising.
It was concluded that for patients with Relapsed/Refractory Mantle Cell Lymphoma, CALQUENCE® given as a single agent resulted in a high and durable Objective Response Rate as well as Complete Response Rate, with a favorable safety profile. CALQUENCE® is a new treatment option for this aggressive malignancy. Efficacy and Safety of Acalabrutinib Monotherapy in Patients with Relapsed/Refractory Mantle Cell Lymphoma in the Phase 2 ACE-LY-004 Study. Wang M, Rule S, Zinzani PL, et al. 59th Annual Meeting & Exposition Atlanta, GA. December 9-12, 2017. #155
CALQUENCE® (Acalabrutinib)
The FDA on October 31, 2017 granted accelerated approval to CALQUENCE® for treatment of adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. CALQUENCE® is a product of AstraZeneca Pharmaceuticals Inc. under license of Acerta Pharma BV.
YESCARTA® (Axicabtagene ciloleucel)
The FDA on October 18, 2017 granted regular approval to YESCARTA®, for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including Diffuse Large B-Cell Lymphoma (DLBCL) Not Otherwise Specified, Primary Mediastinal large B-cell Lymphoma, high-grade B-cell lymphoma, and DLBCL arising from Follicular lymphoma. YESCARTA® is a product of Kite Pharma, Inc.
FDA Approves ALIQOPA®, a PI3K Inhibitor, for Follicular Lymphoma
The FDA in September 2017, granted accelerated approval to ALIQOPA® (Copanlisib) for the treatment of adult patients with relapsed Follicular Lymphoma, who have received at least two prior systemic therapies. Follicular Lymphoma is the most indolent form and second most common form of all Non Hodgkin Lymphomas and approximately 30% of the patients will relapse in 3 years following initial treatment. ALIQOPA® is a pan-class 1, PI3K inhibitor with inhibitory activity predominantly against PI3K-α and PI3K-δ Isoforms expressed in malignant B cells. ALIQOPA® has significant activity in patients with relapsed/refractory indolent B-cell lymphoma, and the safety was manageable, compared with other PI3K inhibitors.
FDA Approves CAR T-Cell Therapy for Non Hodgkin Lymphoma
SUMMARY: The FDA on October 18, 2017, granted regular approval to Axicabtagene ciloleucel (YESCARTA®) for the treatment of adult patients with relapsed or refractory Large B-Cell Lymphoma after two or more lines of systemic therapy, including Diffuse Large B-Cell Lymphoma (DLBCL) Not Otherwise Specified, Primary Mediastinal Large B-Cell Lymphoma, High-grade B-Cell Lymphoma, and DLBCL arising from Follicular Lymphoma (Transformed Follicular Lymphoma-TFL).
What is (CAR) T-cell immunotherapy? Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy and consists of T cells collected from the patient’s blood in a leukapheresis procedure, and genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors (CAR). These reprogrammed cytotoxic T cells with the Chimeric Antigen Receptors on their surface are now able to recognize a specific antigen on tumor cells. These genetically engineered and reprogrammed CAR T-cells are grown in the lab and are then infused into the patient. These cells in turn proliferate in the patient’s body and the engineered receptor on the cell surface help recognize and kill cancer cells that expresses that specific antigen. It is a therefore a customized treatment created using patient’s own T cells to destroy cancer cells.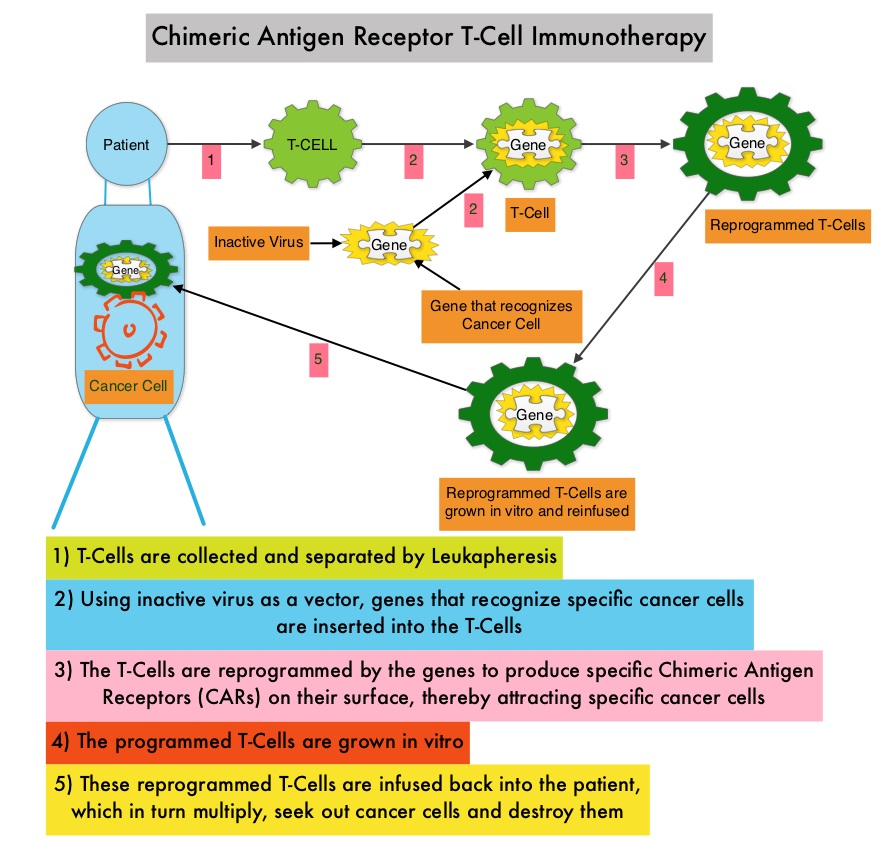
YESCARTA® is a Chimeric Antigen Receptor (CAR) T cell immunotherapy and consists of autologous T cells that are genetically modified to produce a CAR protein, allowing the T cells to seek out and destroy cancer cells expressing the antigen CD19, which is found uniquely on B cells. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome (CRS), an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the central nervous system and eradicate cancer cells. CD19 antigen is expressed by majority of the B cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL) and Non Hodgkin lymphoma (NHL), such as Diffuse Large B-Cell Lymphoma (DLBCL).
Diffuse Large B-Cell Lymphoma (DLBCL) is the most common of the aggressive Non-Hodgkin lymphoma’s in the United States, and the incidence has steadily increased 3 to 4% each year. Outcomes for patients with relapsed/refractory disease is poor, with an Objective response Rate (ORR) of 26%, Complete Response (CR) rate of 8% and a median Overall Survival (OS) of 6.6 months. There is therefore a significant unmet need in this patient group.
The safety and efficacy of YESCARTA® was evaluated in a single arm multicenter clinical trial (ZUMA-1 study) in which 111 patients were enrolled and 101 patients received therapy with YESCARTA®. This study included patients with DLBCL (N=77) and Primary Mediastinal B-Cell Lymphoma (PMBCL)/Transformed Follicular Lymphoma-TFL (N=24), with chemo refractory disease or patients who had relapsed within 12 months post Autologous Stem Cell Transplantation. Following 3 days of conditioning regimen with Cyclophosphamide and Fludarabine, patients received a single infusion of YESCARTA® at a dose of 2 x 106 CAR-positive T cells/kg. The average turnaround time from apheresis to delivery to clinical site for infusion, was 17 days. The Primary end point was Objective Response Rate (ORR) and Secondary endpoints included Duration of Response, Overall Survival and Safety. The preliminary results were reported after the Primary end point was met.
After a median follow up of 8.7 months, the ORR for the entire group was 82% with 54% CR rate (P<0.0001). Among those with DLBCL, the ORR was 82% and the CR rate was 49% and in the PMBCL/TFL group, the ORR was 83% and the CR rate was 71%. At the time of median follow up, 44% of the patients had ongoing responses and the median Duration of Response was 8.2 months and not reached for those in CR. The median Progression Free Survival was 5.9 months and median OS was not reached.
The most common grade 3 or higher adverse reactions included cytopenias, febrile neutropenia, fever, Cytokine Release Syndrome (CRS) and neurologic events. CRS and neurologic events were generally reversible and 43% received Tocilizumb and 27% received steroids and this did not negatively impact outcomes.
The authors concluded that ZUMA-1 is the first pivotal trial of CD19-specific CAR T-cell therapy in patients with refractory aggressive Non Hodgkin Lymphoma, demonstrating significant clinical activity, with a Complete Response rate 7 times higher than the historic control rate. Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (Axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Locke FL, Neelapu SS, Bartlett NL, et al. Presented at: 2017 AACR Annual Meeting; April 1-5, 2017; Washington, DC. Abstract CT019.
GAZYVA® Superior to RITUXAN® for First-Line Treatment of Follicular Lymphoma
SUMMARY: The American Cancer Society estimates that in 2017, about 72,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,140 individuals will die of this disease. Indolent Non Hodgkin Lymphomas are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas. Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab). This can result in a median Progression Free Survival (PFS) of 6-8 yrs and a median Overall Survival of 12-15 yrs. However, approximately 30% of the patients will relapse in 3 years.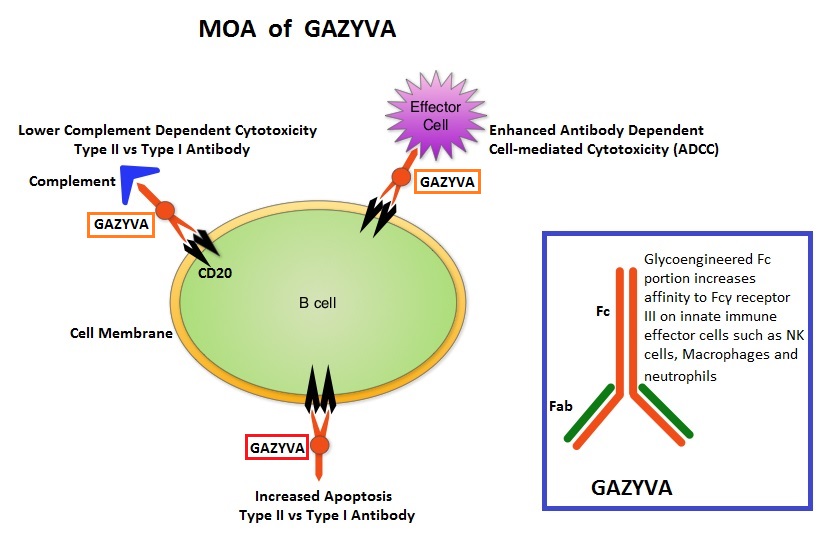
GAZYVA® (Obinutuzumab) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells (natural killer cells, macrophages and neutrophils), Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and Antibody-Dependent Cellular phagocytosis are significantly enhanced, but induces very little Complement-Dependent Cytotoxicity. This is in contrast to RITUXAN® which is a first generation type I, chimeric, anti-CD20 targeted monoclonal antibody that kills lymphoma cells primarily by Complement-Dependent Cytotoxicity and also ADCC.
GAZYVA® along with Bendamustine in the phase III GADOLIN study prolonged PFS, compared with Bendamustine alone, in patients with relapsed/refractory indolent Non Hodgkin lymphoma. Based on this promising data, the GALLIUM phase III trial was conducted in treatment naïve patients with Follicular Lymphoma. This study included 1,202 patients with newly diagnosed Follicular Lymphoma, who had Grade I-IIIa tumors and had an ECOG PS of 2 or less. Patients were randomized to receive either GAZYVA® plus chemotherapy, followed by GAZYVA® maintenance (N=601), or RITUXAN® plus chemotherapy, followed by RITUXAN® maintenance (N=601). The chemotherapy regimens used were CHOP, CVP or Bendamustine, based on the discretion of the treating physician. Patients received either RITUXAN® 375mg/m2 IV on day 1 of each cycle or GAZYVA® 1000 mg IV on days 1, 8, and 15 of cycle 1 and day 1 of subsequent cycles, for either eight 21-day cycles (CHOP and CVP) or six 28-day cycles (Bendamustine). Patients who achieved a Complete Response (CR) or Partial Response (PR) at the end of induction therapy, received maintenance therapy with RITUXAN® or GAZYVA® every 2 months for 2 years or until disease progression. The median age was 59 years and 57.1% of patients received Bendamustine, 33.1% received CHOP, and 9.8% received CVP. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Response Rate, Overall Survival (OS), Disease Free Survival and safety. After a median follow up of 34.5 months, upon recommendations from the Independent Monitoring Committee, the study was unblinded after a preplanned interim efficacy analysis.
The estimated 3-year rate of Progression Free Survival in the GAZYVA® group was 80% compared with 73.3% in the RITUXAN® group, with a 34% reduction in the risk of progression or death noted in the GAZYVA® group (HR=0.66; P=0.001). There was however no difference between the two treatment groups in the 3-year Overall Survival (OS) rate (P=0.21). There was also no difference in the Response Rates between the two treatment groups ((88.5% in the GAZYVA® group and 86.9% in the RITUXAN® group). Patients treated with GAZYVA® had more serious adverse events, 46.1% versus 39.9% in the RITUXAN® group, but the discontinuation rate was similar in both treatment groups.
The authors concluded that for treatment naïve Follicular Lymphoma patients, combining GAZYVA® with chemotherapy resulted in a clinically meaningful improvement in PFS compared with RITUXAN® plus chemotherapy. Whether the improved Progression Free Survival in the GAZYVA® group is related to the maintenance treatment, remains to be explored. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. Marcus R, Davies A, Ando K, et al. N Engl J Med 2017; 377:1331-1344
ALIQOPA® (Copanlisib)
The FDA on September 14, 2017 granted accelerated approval to ALIQOPA®, for the treatment of adult patients with relapsed Follicular Lymphoma, who have received at least two prior systemic therapies. ALIQOPA® is a product of Bayer HealthCare Pharmaceuticals Inc.
FDA Approves ALIQOPA®, a PI3K Inhibitor, for Follicular Lymphoma
SUMMARY: The FDA on September 14, 2017, granted accelerated approval to ALIQOPA® (Copanlisib) for the treatment of adult patients with relapsed Follicular Lymphoma, who have received at least two prior systemic therapies. The American Cancer Society estimates that in 2017, about 72,240 people will be diagnosed with Non Hodgkin Lymphoma (NHL) in the United States and about 20,140 individuals will die of this disease. Indolent NHLs are mature B cell lymphoproliferative disorders and include Follicular Lymphoma, Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT), Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Approximately 20% of all NHLs are Follicular Lymphomas. Advanced stage indolent NHL is not curable and as such, prolonging Progression Free Survival (PFS) and Overall Survival (OS), while maintaining Quality of Life, have been the goals of treatment intervention. Asymptomatic patients with indolent NHL are generally considered candidates for “watch and wait” approach whereas those with B symptoms (fever, night sweats, and weight loss), painful lymphadenopathy/splenomegaly, organ compromise and cytopenias are generally considered candidates for therapy.. Follicular Lymphoma International Prognostic Index (FLIPI) is of prognostic value and is used to help with treatment choices. The Ann Arbor classification divides FL into four stages. Patients with stages I and II have localized disease and those with stages III and IV have advanced disease. The World Health Organization (WHO) further classified FL based on histology into low grade (grades 1 and 2) and high grade (grade 3a) FLs. Grade 3b FL which demonstrates diffuse areas of involvement is designated as Diffuse Large B-cell Lymphoma (DLBCL) and is treated as such. Patients with advanced stage symptomatic Follicular Lymphoma are often treated with induction chemoimmunotherapy followed by maintenance RITUXAN® (Rituximab). This can result in a median PFS of 6-8 yrs and a median Overall Survival of 12-15 yrs. However, approximately 30% of the patients will relapse in 3 years and treatment options are limited for patients with relapses, after multiple treatments.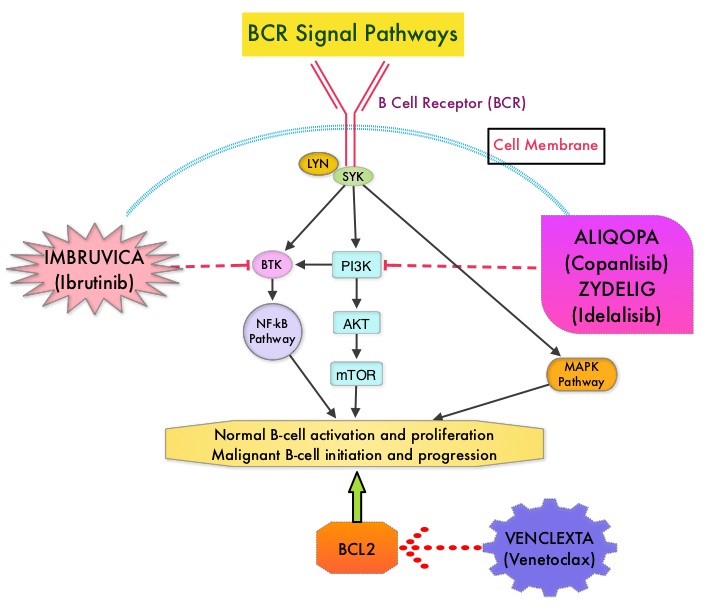
ALIQOPA® is a pan-class 1, PI3K inhibitor with inhibitory activity predominantly against PI3K-α and PI3K-δ Isoforms expressed in malignant B cells. The alpha isoform is broadly expressed and involved in insulin signaling and angiogenesis, as well as resistance mechanisms to lymphoma whereas the delta isoform is expressed by leukocytes and is involved in B-cell signaling, development, and survival. ALIQOPA® has been shown to induce tumor cell death by apoptosis and inhibition of proliferation of primary malignant B cell lines. ALIQOPA® also inhibits several key cell-signaling pathways, including B-cell receptor (BCR) signaling, CXCR12 (C-X-C chemokine receptor 12) mediated chemotaxis of malignant B cells, and NFÏ°B (Nuclear Factor Kappa B) signaling in lymphoma cell lines.
The approval of ALIQOPA® was based on data from the CHRONOS-1 trial, which is an open-label, single arm, multicenter, phase II study of patients with relapsed, Indolent or aggressive Non Hodgkin Lymphomas. This trial included patients with Follicular lymphoma (Grades 1-3a), Marginal Zone Lymphoma, Small Lymphocytic Lymphoma, and LymphoPlasmacytic Lymphoma /Waldenstrom Macroglobulinemia . Eligible patients had relapsed or refractory disease and had received at least two prior systemic therapies. The efficacy data leading to the FDA approval included 104 patients with Follicular B-cell Non Hodgkin Lymphoma. In this trial, patients received 0.8 mg/kg or 60 mg of ALIQOPA® by IV infusion on days 1, 8, and 15 of a 28-day treatment cycle, until disease progression or development of unacceptable toxicity. The median patient age was 63 yrs and all study patients had prior exposure to RITUXAN® and one or more alkylating agents, and 60% of the patients had disease that was refractory to the last regimen received. The Primary endpoint was Objective Response Rate after a minimum of 16 weeks of treatment. Secondary endpoints included Progression Free Survival, Duration of Response, Overall Survival, safety, and Quality of Life.
The Objective Response Rate was 58.7%, with an estimated median response duration of 12.2 months. The Complete Response rate was 14.4% and partial response rate was 44.3% and 33.7% has stable disease. The most common adverse reactions included nausea, hyperglycemia, diarrhea, fatigue, hypertension, cytopenias and lower respiratory tract infections. According to the authors, safety was manageable compared with the other PI3K inhibitor approved by the FDA, ZYDELIG® (Idelalisib), which targets only the δ-isoform and has warnings against fatal or severe colitis, intestinal perforation, hepatotoxicity, and pneumonitis The safety advantage with ALIQOPA® may be due to the dose scheduling or the IV mode of delivery.
It was concluded that ALIQOPA® has significant activity in patients with relapsed/refractory indolent B-cell lymphoma, and the safety was manageable, compared with other PI3K inhibitors. Two phase III trials are underway, using ALIQOPA® in combination with RITUXAN®. Dreyling M, Santoro A, Mollica L, et al. Copanlisib in patients with relapsed or refractory indolent B-cell lymphoma: Primary results of the pivotal CHRONOS-1 study. Presented at: 2017 AACR Annual Meeting; April 1-5, 2017; Washington, DC. Abstract CT149.
