The FDA on November 10, 2015 approved COTELLIC® Tablets for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutation, in combination with Vemurafenib. COTELLIC® is not indicated for treatment of patients with wild-type BRAF melanoma. COTELLIC® is a product of Genentech, Inc.
Tag: Malignant Melanoma of the Skin
IMLYGIC® (Talimogene laherparepvec)
The FDA on October 27, 2015 approved IMLYGIC®, a genetically-modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma, recurrent after initial surgery. IMLYGIC® is a product of Amgen, Inc.
BRAF Inhibitors versus Immunotherapy in Patients with BRAF V600-Mutant Metastatic Melanoma
SUMMARY: It is estimated that in the US, approximately 76,380 new cases of melanoma will be diagnosed in 2016 and approximately 10,130 patients will die of the disease. The incidence of melanoma has been on the rise for the past three decades. The approval of the combination of MEKINIST® (Trametinib) and TAFINLAR® (Dabrafenib), to treat patients with advanced melanoma, was based on the understanding of the biological pathways of this malignancy. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6%-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas. In the BREAK-3 randomized phase III trial, TAFINLAR® (Dabrafenib), a selective oral BRAF inhibitor demonstrated a statistically significant improvement in Progression Free Survival (PFS) and Response Rate (RR) compared to Dacarbazine (DTIC), in patients with advanced BRAF V600E/K mutated melanoma. However, Squamous Cell carcinomas were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation (BRAF wild-type cells) has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. The addition of a MEK inhibitor such as MEKINIST® (Trametinib) to a BRAF inhibitor such as TAFINLAR®, has addressed some of these limitations, in previously published studies, with improvement in PFS. MEKINIST® is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway and has been shown to significantly improve PFS, RR and Overall Survival (OS), when compared to chemotherapy, in advanced melanoma patients with BRAF V600E/K mutations. A combination of BRAF inhibitor TAFINLAR® and MEK inhibitor MEKINIST®, significantly improved OS, as compared with monotherapy with BRAF inhibitor, with a 31% relative reduction in the risk of death, in previously untreated patients with metastatic melanoma, with BRAF V600E or V600K mutations. This benefit was accomplished without increased overall toxicity. However, approximately 50% of patients progress after 12 months, although a significant number of patients experience long-term benefit without progression.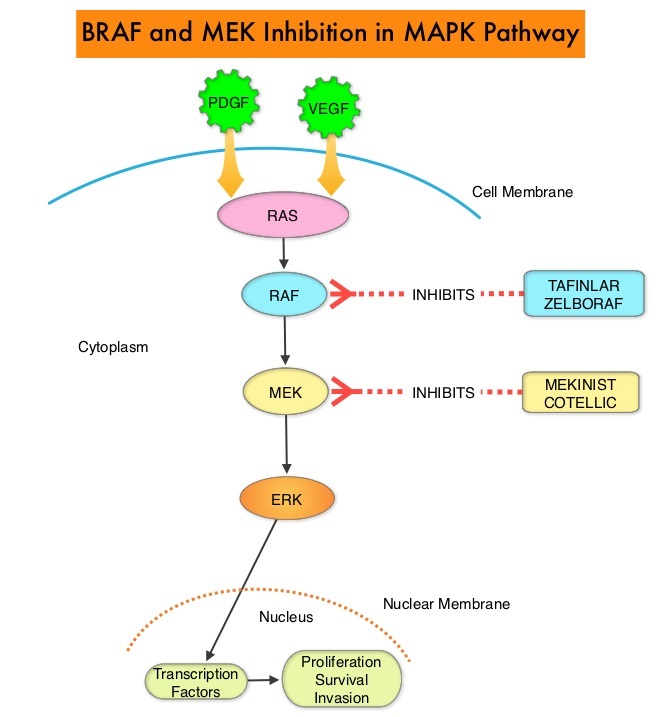
The purpose of this study was to identify the clinical predictors and analyze the clinical correlates of those who had prolonged survival. The authors in this manuscript report the updated OS results of a previously published study, in which BRAF inhibitor-naive patients were treated with a combination of TAFINLAR® and MEKINIST®. Additionally, the authors in this study also report the clinical factors associated with long term survival. The original open-label phase I and II study of combination therapy with TAFINLAR® and MEKINIST® (N Engl J Med. 2012;367:1694-1703) had four parts (parts A, B, C, and D). The present analysis evaluated the outcomes of a total of 78 BRAF inhibitor-naive patients, enrolled in part B (N= 24) and part C (N= 54), who received the phase III dose (optimal dose) of oral TAFINLAR® 150 mg twice daily combined with oral MEKINIST® 2 mg once daily. The remaining cohorts in parts B and C and all cohorts in parts A and D did not receive the phase III dose of the combination therapy and therefore are not described here.
It was noted that among patients in part B of the study, the 1 year Progression Free Survival (PFS) was 44%, 2 year PFS was 22% and 3 year PFS was 18%. Among patients in part C, the 1 year PFS was 41%, 2 year PFS was 25% and 3 year PFS was 21%. The median Overall Survival (OS) was 27.4 months in part B and 25 months in part C with an OS at 1, 2, and 3 years of 72%, 60%, and 47%, respectively, for part B and 80%, 51%, and 38%, respectively, for part C. Prolonged survival was associated with good prognostic factors such as metastases in fewer than three organ sites and lower baseline LDH (Lactate Dehydrogenase) levels. The 3 year OS was 62% in patients with normal baseline LDH levels and 63% in those who had a complete response.
The authors concluded that a combination of TAFINLAR® and MEKINIST® results in a median OS of more than 2 years in BRAF V600 mutation-positive metastatic melanoma, with approximately 20% of the patients remaining progression free at 3 years. Good prognostic features at baseline, were predictive of durable responses. With dilemma facing clinicians, whether to choose MAP Kinase inhibitors versus Immunotherapy, as first line therapy for this patient group, the longest follow up data presented, demonstrating durable benefit with a combination TAFINLAR® and MEKINIST®, should be very reassuring. Overall Survival and Durable Responses in Patients With BRAF V600-Mutant Metastatic Melanoma Receiving Dabrafenib Combined With Trametinib. Long GV, Weber JS, Infante JR, et al. JCO JCO629345; published online on January 25, 2016
FDA Approves COTELLIC® in Combination with ZELBORAF® for Advanced Melanoma
SUMMARY: The U.S. FDA on November 10, 2015, approved COTELLIC® (Cobimetinib) for the treatment of patients with unresectable or metastatic melanoma, with a BRAF V600E or V600K mutation, in combination with ZELBORAF® (Vemurafenib). The American Cancer Society estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway, which enables the cell to respond to external stimuli. This pathway plays a dual role regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas. In the BRIM 3 randomized, phase III study, ZELBORAF® (Vemurafenib), a selective oral inhibitor of mutated BRAF demonstrated significant improvement in Progression Free Survival and Overall Survival compared to Dacarbazine. Squamous cell carcinoma’s were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. MEK gene is downstream from RAF in the MAPK pathway. The addition of a selective inhibitor of MEK gene such as COTELLIC® (Cobimetinib) to a BRAF inhibitor such as ZELBORAF®, has addressed some of these limitations, in previously published studies, with improvement in Objective Response rates and decrease in the incidence of cutaneous secondary cancers.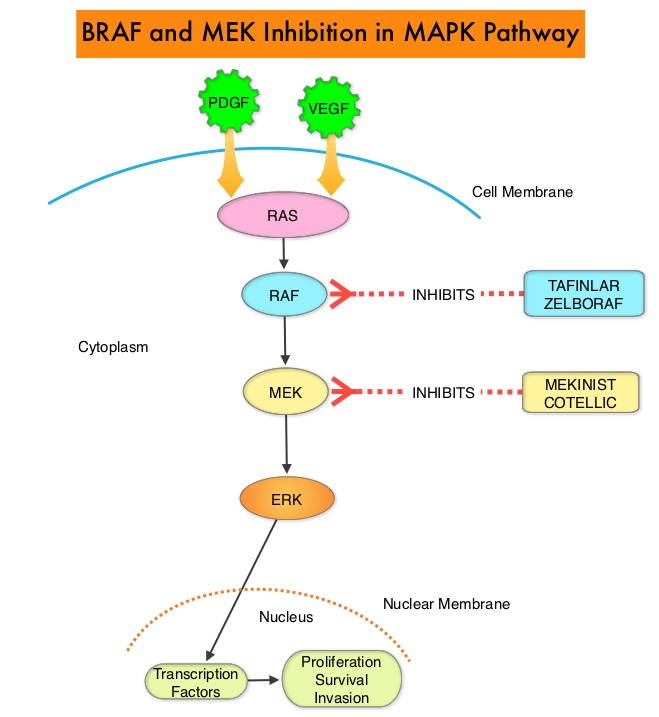
coBRIM is an international, multicenter, randomized, phase III study in which the efficacy and safety of COTELLIC® combined with ZELBORAF®, was evaluated in previously untreated patients, with advanced BRAF-mutated melanoma. Four hundred and ninety five (N=495) patients were randomly assigned in a 1:1 ratio to receive ZELBORAF® 960 mg orally twice daily along with either COTELLIC® 60 mg orally once daily on days 1-21 (N=247) or matching placebo (N=248), of a 28 day cycle. BRAF V600 mutation-positive status was detected using the cobas 4800 BRAF V600 mutation test. The median age of the study group was 55 years and patient demographics in both treatment groups were well balanced. About 60% of the patients, had stage IV disease. The primary endpoint for the study was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Objective Response Rate (ORR), and duration of response.
The primary analysis published in NEJM demonstrated a significant improvement in the median PFS (9.9 months vs 6.2 months) as well as ORR (68% vs 45%) with the combination of ZELBORAF® and COTELLIC® compared to ZELBORAF® and placebo respectively. In this updated analysis submitted to the FDA, the median PFS with the combination of ZELBORAF® and COTELLIC® was 12.3 versus 7.2 months for ZELBORAF® alone (HR= 0.56; P <0.001). There was also a statistically significant improvement in OS based on an interim analysis, with the median OS not reached (NR) in the combination group versus 17 months in the single agent ZELBORAF® group (HR=0.63; P=0.0019). The ORR were 70% and 50% in the ZELBORAF® and COTELLIC® and single agent ZELBORAF® groups, respectively (P<0.001).
The most common adverse reactions were diarrhea, photosensitivity reaction, nausea, pyrexia and vomiting. Treatment-related discontinuation rates in the combination and single agent groups were similar at 13% and 12%, respectively. It was concluded that in patients with unresectable or metastatic melanoma, with a BRAF V600E or V600K mutation, a combination of COTELLIC® and ZELBORAF® delays disease progression and improves survival compared to single agent ZELBORAF®. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: Phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. Larkin JMG, Yan Y, McArthur GA, et al. J Clin Oncol. 2015;33 (suppl; abstr 9006).
YERVOY® (Ipilimumab)
The FDA on October 28, 2015 approved YERVOY® injection for the additional indication of adjuvant treatment of patients with Cutaneous Melanoma, with pathologic involvement of regional lymph nodes of more than 1 mm, who have undergone complete resection, including total lymphadenectomy. YERVOY® is a product of Bristol-Myers Squibb Company.
IMLYGIC® a Novel Oncolytic Immunotherapy Demonstrates Significant Responses in Advanced Melanoma
SUMMARY: The FDA on October 27, 2015 approved IMLYGIC® (Talimogene laherparepvec or T-VEC), the first FDA-approved oncolytic virus therapy, for the treatment of melanoma lesions in the skin and lymph nodes. The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. IMLYGIC® is a genetically modified, herpes simplex virus type 1–derived oncolytic immunotherapy designed to induce both local and systemic immune responses. Following injection directly into melanoma lesions, IMLYGIC® selectively replicates within tumors and produces an immunostimulatory protein called Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). IMLYGIC® causes cell lysis resulting in the release of tumor-derived antigens, which along with the local GM-CSF, recruit and activate antigen-presenting cells, with subsequent induction of tumor-specific T-cell responses. The enhanced systemic antitumor immune response against tumor-derived antigens, eradicates tumor cells elsewhere in the body.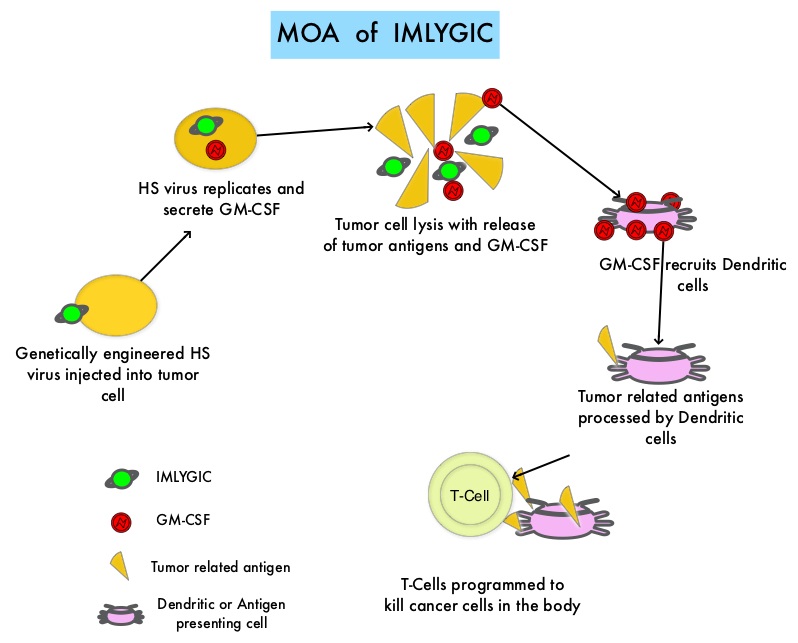
Previously reported single arm phase II study with IMLYGIC® demonstrated an Overall Response Rate (ORR) of 26% in patients with stage IIIC to IV melanoma, with responses noted in both injected and un-injected lesions, including visceral lesions. Oncovex (GM-CSF) Pivotal Trial in Melanoma (OPTiM) is a phase III study in which 436 patients with stage IIIB to IV melanoma, with injectable melanoma lesions that could not be surgically resected, were randomly assigned in a 2:1 ratio to receive intralesional IMLYGIC® (N=295) or subcutaneous GM-CSF (N=141). The enrolled patient’s melanoma lesions in the skin and lymph nodes were treated with IMLYGIC® or a comparator therapy and Injection into visceral lesions was not allowed. Patients in the IMLYGIC® group received a series of injections into the melanoma lesions. Following the initial injection, a second dose was administered three weeks later, followed by additional doses every two weeks for at least six months, unless other treatment was required or until there are were no remaining injectable lesions to treat. Patients in the GM-CSF group received 125 micrograms/m2 subcutaneously once daily for 14 days in 28-day cycles. The median age of patients in the study was 63 years. The primary end point was Durable Response Rate (DRR- Objective Response lasting continuously for 6 months or more). Secondary end points included Overall Survival (OS) and Overall Response Rate.
The DRR was significantly higher among patients receiving IMLYGIC® than among those given GM-CSF (16.3% vs 2.1%; P<0.001). The Overall Response Rate was also higher with IMLYGIC® compared to GM-CSF (26.4% vs 5.7%; P<0.001). Approximately 11% of patients receiving IMLYGIC® experienced a Complete Response compared to less than 1% for those receiving GM-CSF. The median Time to Treatment Failure was 8.2 months with IMLYGIC® and 2.9 months with GM-CSF (HR=0.42) and median Overall Survival was 23.3 months and 18.9 months, respectively (HR=0.79; P=0.051). In an exploratory subset analysis, the benefit with IMLYGIC® was more pronounced among patients with stage IIIB, IIIC, or IV M1a disease, as well as among patients who were treatment naïve. The most common side effects were fatigue, chills, fever, nausea, flu-like symptoms and pain at the injection site. IMLYGIC® should not be given to individuals with suppressed immune systems or who are pregnant because IMLYGIC® is a modified live oncolytic herpes virus therapy and herpes virus infection can potentially occur.
The authors concluded that IMLYGIC® is the first oncolytic immunotherapy to demonstrate therapeutic benefit in patients with advanced unresectable melanoma and may be another novel therapeutic option for patients with metastatic melanoma. Combining IMLYGIC® with T-Cell checkpoint inhibitor, YERVOY® (Ipilimumab) is presently being explored and thus far has shown encouraging results with minimal added toxicities. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Andtbacka RH, Kaufman HL, Collichio F, et al. Published online before print May 26, 2015, doi: 10.1200/JCO.2014.58.3377
YERVOY® and OPDIVO® Combination Superior to YERVOY® Monotherapy in Advanced Melanoma
SUMMARY: The FDA on September 30, 2015 granted accelerated approval to OPDIVO® (Nivolumab) in combination with YERVOY® (Ipilimumab), for the treatment of patients with BRAF V600 wild-type, unresectable or metastatic melanoma. The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The understanding of the Immune checkpoints has lead to the development of novel immunotherapies. Immune checkpoints or gate keepers are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), PD-1(Programmed cell Death-1), etc. By doing so, the T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response. YERVOY® is a fully human immunoglobulin G1 monoclonal antibody, that blocks Immune checkpoint protein/receptor CTLA-4 and has been shown to prolong Overall Survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response. OPDIVO® significantly improves Objective Response Rates, in patients with advanced melanoma. Previously published phase I studies demonstrated high Objective Response Rates, including Complete Responses, among patients with advanced melanoma, when treated with a combination of YERVOY® and OPDIVO®.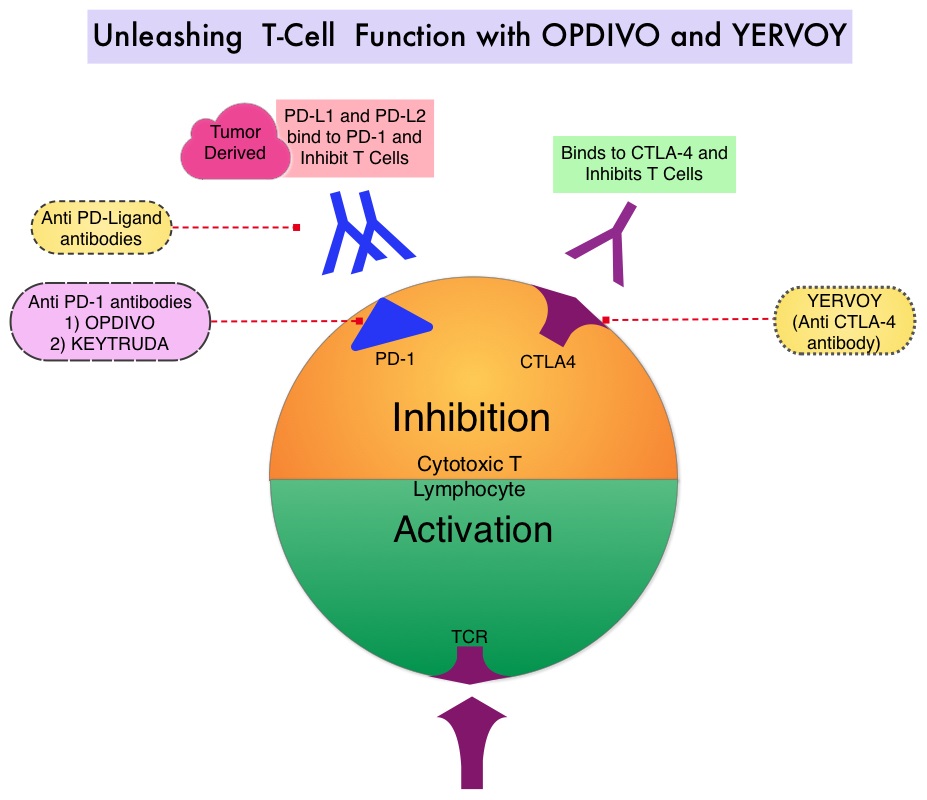
In this double-blind phase II trial, 142 treatment naïve patients with metastatic melanoma were randomly assigned in a 2:1 ratio to receive either OPDIVO® in combination with YERVOY® (N=95) or YERVOY® plus placebo (N=47). Patients in the OPDIVO® plus YERVOY® group received OPDIVO® 1 mg/kg and YERVOY® 3 mg/kg IV every 3 weeks for four doses followed by OPDIVO® 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. Patients in the YERVOY® and placebo group received YERVOY® 3 mg/kg and placebo IV every 3 weeks for four doses followed by placebo. At the time of disease progression, patients in the YERVOY® group were offered OPDIVO® 3 mg/kg every 2 weeks. Patients were stratified according to BRAF mutation status (wild type versus mutation-positive). The median age was 65 years and majority of patients were males. The primary end point was Objective Response Rate among patients with BRAF V600 wild type tumors. The authors noted that in BRAF wild type tumors, the overall Response Rate was 61% with the combination treatment versus 11% for YERVOY® alone (P<0.001). The Complete Response rate was 22% for the combination therapy vs 0% for YERVOY®. The median duration of response was not reached in either treatment groups, with an ongoing response rate of 82% in the combination group and 75% in the YERVOY® plus placebo group. The median Progression Free Survival was not reached with the combination therapy and was 4.4 months in the YERVOY® plus placebo group (HR=0.40; P<0.001). Among patients with BRAF mutation-positive tumors, the overall Response Rate was 52% for the combination therapy group and Complete Response rate was 22%, with similar efficacy compared with the BRAF wild type tumor group. The median Progression Free Survival was 8.5 months for the combination therapy group and 2.7 months for YERVOY® plus placebo group.
Grade 3 or 4 adverse events were reported in 54% of the patients who received the combination therapy as compared with 24% of the patients who received YERVOY® monotherapy and the most common grade 3 or 4 toxicities in the combination therapy group were colitis, diarrhea and elevated transaminases, which resolved with steroids. The authors concluded that a combination of YERVOY® and OPDIVO® is superior to YERVOY® monotherapy, among treatment naïve patients with metastatic melanoma, with significantly greater objective response rate, as well as Progression Free Survival. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. Postow MA, Chesney J, Pavlick AC, et al. N Engl J Med 2015; 372:2006-2017
OPDIVO® (Nivolumab)
The FDA on September 30, 2015 granted accelerated approval to OPDIVO® in combination with YERVOY® (Ipilimumab), for the treatment of patients with BRAF V600 wild-type, unresectable or metastatic melanoma. OPDIVO® Injection is a product of Bristol-Myers Squibb Company.
Long Term Survival with YERVOY® in Advanced Malignant Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The incidence of melanoma has been on the rise for the past 30 years. The US Food and Drug Administration approved YERVOY® (Ipilimumab) for the treatment of unresectable or metastatic melanoma in March 2011. This therapy was the first, to improve Overall Survival in a phase III trial. YERVOY® is a fully human immunoglobulin G1 monoclonal antibody, that blocks Immune checkpoint protein/receptor CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), and this is the first Immune checkpoint protein that was clinically targeted.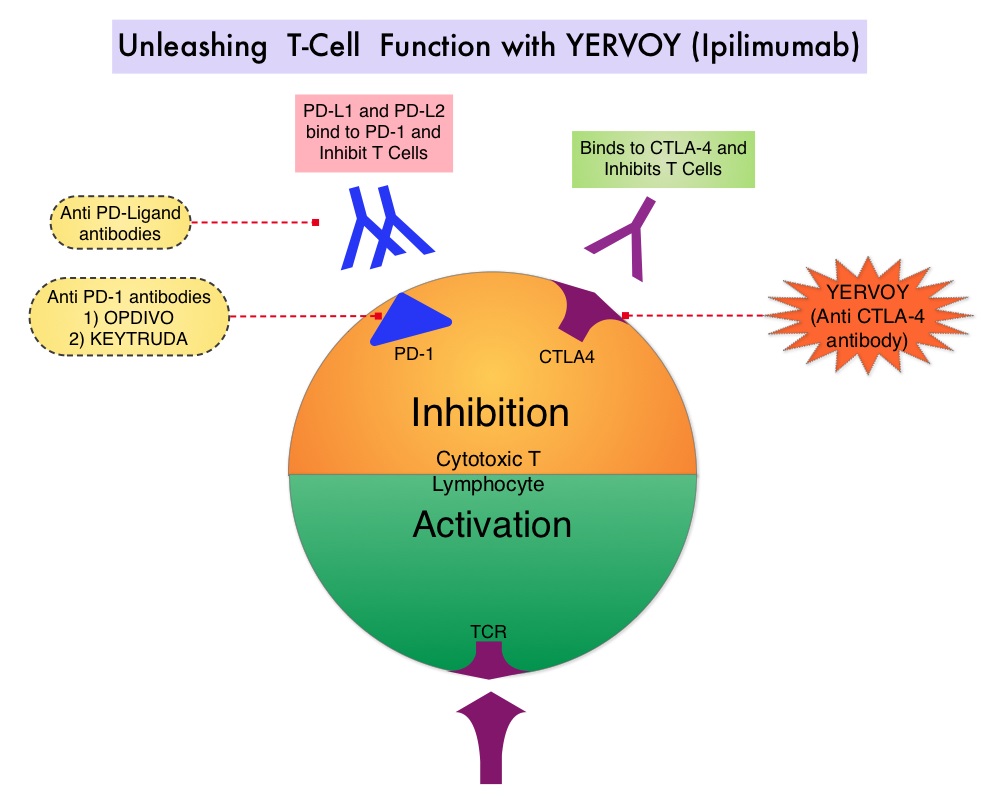 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4, also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. CA184-024 is a phase III trial in which treatment naïve patients with stage IIIc, N3 (unresectable), or stage IV melanoma were randomly assigned to receive YERVOY® 10 mg/kg IV given along with Dacarbazine 850 mg/m2 IV (N=250) or placebo plus Dacarbazine 850 mg/m2 IV (N=252) administered every 3 weeks for 4 doses followed by Dacarbazine given alone every 3 weeks through week 22. Responders and those with stable disease from week 12 through week 24 were then allowed to receive YERVOY® or placebo as maintenance therapy given every 12 weeks beginning at week 24, until disease progression or unacceptable toxicity. Dacarbazine was not given during the maintenance phase. The median Overall Survival was significantly longer in the group treated with YERVOY® plus Dacarbazine than in patients treated with placebo plus Dacarbazine (11.2 vs 9.1 months, HR=0.72; P< 0.001). This Overall Survival benefit was maintained after 3 years of follow up and toxicities were manageable.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4, also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. CA184-024 is a phase III trial in which treatment naïve patients with stage IIIc, N3 (unresectable), or stage IV melanoma were randomly assigned to receive YERVOY® 10 mg/kg IV given along with Dacarbazine 850 mg/m2 IV (N=250) or placebo plus Dacarbazine 850 mg/m2 IV (N=252) administered every 3 weeks for 4 doses followed by Dacarbazine given alone every 3 weeks through week 22. Responders and those with stable disease from week 12 through week 24 were then allowed to receive YERVOY® or placebo as maintenance therapy given every 12 weeks beginning at week 24, until disease progression or unacceptable toxicity. Dacarbazine was not given during the maintenance phase. The median Overall Survival was significantly longer in the group treated with YERVOY® plus Dacarbazine than in patients treated with placebo plus Dacarbazine (11.2 vs 9.1 months, HR=0.72; P< 0.001). This Overall Survival benefit was maintained after 3 years of follow up and toxicities were manageable.
The authors in this publication conducted a milestone survival analysis with a minimum follow-up of 5 years and they noted that the 5-year survival rate doubled and was 18.2% for patients treated with YERVOY® plus Dacarbazine versus 8.8% for patients treated with placebo plus Dacarbazine (P=0.002). The plateau in the survival curve began at approximately 3 years. In patients who survived at least 5 years and continued maintenance YERVOY®, grade 3 or 4 immune-related adverse events were observed exclusively in the skin. Based on these findings, it was concluded that patients treated with YERVOY® for advanced melanoma, continue to respond following a period of stable disease and these ongoing and durable responses may contribute to long term survival in some patients. Five-Year Survival Rates for Treatment-Naive Patients With Advanced Melanoma Who Received Ipilimumab Plus Dacarbazine in a Phase III Trial. Maio M, Grob J, Aamdal S, et al. J Clin Oncol 2015;33: 1191-1196
Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib
SUMMARY: It is estimated that in the US, approximately 76,000 new cases of melanoma were diagnosed and close to 8000 individuals died of the disease, in 2014. The incidence of melanoma has been on the rise for the past three decades. The FDA granted accelerated approval in January 2014, for a combination of MEKINIST® (Trametinib) and TAFINLAR® (Dabrafenib), to treat patients with advanced melanoma, based on the understanding of the biological pathways of this malignancy. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6%-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas. In the BREAK-3 randomized phase III trial, TAFINLAR® (Dabrafenib), a selective oral BRAF inhibitor demonstrated a statistically significant improvement in Progression Free Survival (PFS) and Response Rate (RR) compared to Dacarbazine (DTIC), in patients with advanced BRAF V600E/K mutated melanoma. In the BRIM 3 randomized, phase III study, ZELBORAF® (Vemurafenib), a selective oral inhibitor of mutated BRAF demonstrated significant improvement in Progression Free Survival and Overall Survival compared to Dacarbazine.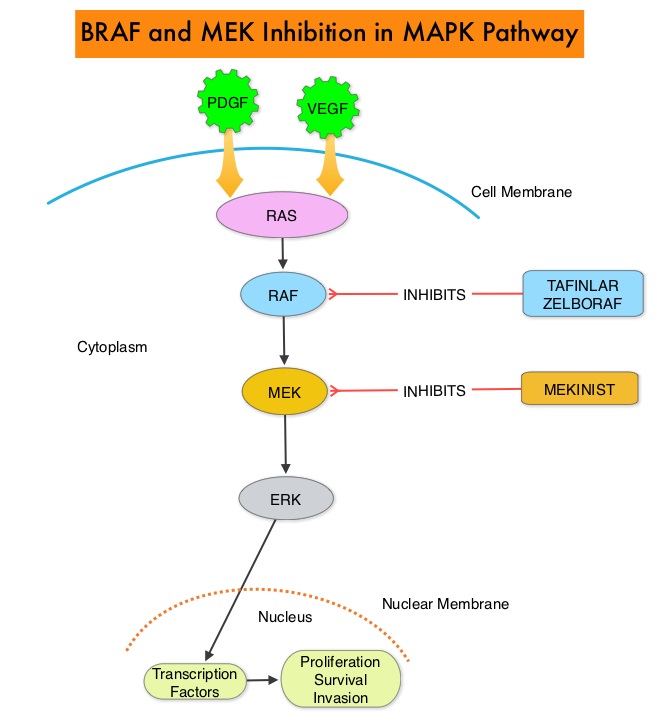 Squamous cell carcinoma’s were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. The addition of a MEK inhibitor such as MEKINIST® (Trametinib) to a BRAF inhibitor such as TAFINLAR®, has addressed some of these limitations, in previously published studies, with improvement in Progression Free Survival. MEKINIST® is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway and has been shown to significantly improve Progression Free Survival, Response Rate and Overall Survival, when compared to chemotherapy, in advanced melanoma patients with BRAF V600E/K mutations. The authors in this open label, randomized, phase III trial, evaluated the outcomes, comparing a combination of TAFINLAR® and MEKINIST® with single agent ZELBORAF® , in previously untreated and unresectable Stage IIIC or IV melanoma patients with BRAF V600E or V600K mutations. Eligible patients (N=704) were assigned in a 1:1 ratio to receive either a combination of TAFINLAR® (Dabrafenib), 150 mg PO BID and MEKINIST ® (Trametinib) 2 mg PO QD or ZELBORAF® (Vemurafenib) 960 mg PO BID, as first line therapy. The primary end point of this study was Overall Survival. Secondary end points included Progression Free Survival, Overall Response Rate, duration of response, and safety. At the preplanned interim analysis, the Overall Survival at 12 months was 72% in the combination therapy group and 65% in the single agent ZELBORAF® group (HR=0.69; P=0.005). The median Progression Free Survival was 11.4 months in the combination therapy group and 7.3 months in the ZELBORAF® group (HR=0.56; P<0.001). The objective response rate was 64% with combination therapy and 51% with single agent ZELBORAF® (P<0.001). There was no difference in the rates of severe adverse events and study drug discontinuations between the two groups. Skin cancers such as Squamous cell carcinoma and Keratoacanthoma occurred in 1% of patients in the combination therapy group and 18% of those treated with ZELBORAF® . The authors concluded that a combination of BRAF inhibitor TAFINLAR® and MEK inhibitor MEKINIST® significantly improved Overall Survival as compared with ZELBORAF® monotherapy, with a 31% relative reduction in the risk of death, in previously untreated patients with metastatic melanoma, with BRAF V600E or V600K mutations. This benefit was accomplished without increased overall toxicity. Robert C, Karaszewska B, Schachter J, et al. N Engl J Med 2015; 372:30-39
Squamous cell carcinoma’s were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. The addition of a MEK inhibitor such as MEKINIST® (Trametinib) to a BRAF inhibitor such as TAFINLAR®, has addressed some of these limitations, in previously published studies, with improvement in Progression Free Survival. MEKINIST® is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway and has been shown to significantly improve Progression Free Survival, Response Rate and Overall Survival, when compared to chemotherapy, in advanced melanoma patients with BRAF V600E/K mutations. The authors in this open label, randomized, phase III trial, evaluated the outcomes, comparing a combination of TAFINLAR® and MEKINIST® with single agent ZELBORAF® , in previously untreated and unresectable Stage IIIC or IV melanoma patients with BRAF V600E or V600K mutations. Eligible patients (N=704) were assigned in a 1:1 ratio to receive either a combination of TAFINLAR® (Dabrafenib), 150 mg PO BID and MEKINIST ® (Trametinib) 2 mg PO QD or ZELBORAF® (Vemurafenib) 960 mg PO BID, as first line therapy. The primary end point of this study was Overall Survival. Secondary end points included Progression Free Survival, Overall Response Rate, duration of response, and safety. At the preplanned interim analysis, the Overall Survival at 12 months was 72% in the combination therapy group and 65% in the single agent ZELBORAF® group (HR=0.69; P=0.005). The median Progression Free Survival was 11.4 months in the combination therapy group and 7.3 months in the ZELBORAF® group (HR=0.56; P<0.001). The objective response rate was 64% with combination therapy and 51% with single agent ZELBORAF® (P<0.001). There was no difference in the rates of severe adverse events and study drug discontinuations between the two groups. Skin cancers such as Squamous cell carcinoma and Keratoacanthoma occurred in 1% of patients in the combination therapy group and 18% of those treated with ZELBORAF® . The authors concluded that a combination of BRAF inhibitor TAFINLAR® and MEK inhibitor MEKINIST® significantly improved Overall Survival as compared with ZELBORAF® monotherapy, with a 31% relative reduction in the risk of death, in previously untreated patients with metastatic melanoma, with BRAF V600E or V600K mutations. This benefit was accomplished without increased overall toxicity. Robert C, Karaszewska B, Schachter J, et al. N Engl J Med 2015; 372:30-39
