The FDA on December 22, 2014 granted accelerated approval to OPDIVO® for the treatment of patients with unresectable or metastatic melanoma and disease progression following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. OPDIVO® is a product of Bristol-Myers Squibb Company.
Tag: Malignant Melanoma of the Skin
A phase 3 randomized, open-label study of nivolumab (anti-PD-1;BMS-936558; ONO-4538) versus investigator's choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy
SUMMARY: The FDA on December 22, 2014 granted accelerated approval to OPDIVO® (Nivolumab) for the treatment of patients with unresectable or metastatic melanoma whose disease has progressed following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2) produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® in previously conducted studies demonstrated durable antitumor activity and promising overall survival (OS) in pretreated patients. CheckMate-037 is an open label, randomized, phase III study, in which 370 patients with unresectable or metastatic melanoma, received OPDIVO® 3 mg/kg IV every 2 weeks (N=268) or investigator’s choice of chemotherapy, which included either Dacarbazine or a combination of Carboplatin plus Paclitaxel given every 3 weeks (N=102). Treatment was continued until disease progression or unacceptable toxicity. Eligible patients were required to have disease progression following YERVOY® (Ipilimumab) and a BRAF inhibitor if BRAF V600 mutation positive. The primary endpoints were ORR and overall survival. Early findings (Objective Response Rate-ORR) in the first 120 patients who were treated with OPDIVO® and in 47 patients treated with chemotherapy and had a minimum 6 months follow up (planned interim analysis), was presented at the 2014 ESMO Congress. The Objective Response Rate (ORR) was 32% in the OPDIVO® group and 11% in the chemotherapy group. The median time to response was 2.1 months in the OPDIVO® group and 3.5 months with chemotherapy. The majority, (95%) of responses at 6 months were ongoing in the OPDIVO® group and the median duration of response was not reached. The most common (greater than or equal to 20%) adverse reaction in the OPDIVO® group was rash. Grade 3 and 4 adverse events were seen in 2-5% of patients receiving OPDIVO® and included abdominal pain, hyponatremia, elevated liver enzymes and increased lipase. Clinically significant immune-mediated adverse reactions were pneumonitis, colitis, hepatitis, nephritis, and thyroid dysfunction. OPDIVO® is a new and novel treatment option for patients with advanced melanoma and is a welcome addition, as we try to better understand tumor immunology. Weber JS, Minor DR, D'Angelo S, et al. ESMO 2014, LBA3_PR
The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® in previously conducted studies demonstrated durable antitumor activity and promising overall survival (OS) in pretreated patients. CheckMate-037 is an open label, randomized, phase III study, in which 370 patients with unresectable or metastatic melanoma, received OPDIVO® 3 mg/kg IV every 2 weeks (N=268) or investigator’s choice of chemotherapy, which included either Dacarbazine or a combination of Carboplatin plus Paclitaxel given every 3 weeks (N=102). Treatment was continued until disease progression or unacceptable toxicity. Eligible patients were required to have disease progression following YERVOY® (Ipilimumab) and a BRAF inhibitor if BRAF V600 mutation positive. The primary endpoints were ORR and overall survival. Early findings (Objective Response Rate-ORR) in the first 120 patients who were treated with OPDIVO® and in 47 patients treated with chemotherapy and had a minimum 6 months follow up (planned interim analysis), was presented at the 2014 ESMO Congress. The Objective Response Rate (ORR) was 32% in the OPDIVO® group and 11% in the chemotherapy group. The median time to response was 2.1 months in the OPDIVO® group and 3.5 months with chemotherapy. The majority, (95%) of responses at 6 months were ongoing in the OPDIVO® group and the median duration of response was not reached. The most common (greater than or equal to 20%) adverse reaction in the OPDIVO® group was rash. Grade 3 and 4 adverse events were seen in 2-5% of patients receiving OPDIVO® and included abdominal pain, hyponatremia, elevated liver enzymes and increased lipase. Clinically significant immune-mediated adverse reactions were pneumonitis, colitis, hepatitis, nephritis, and thyroid dysfunction. OPDIVO® is a new and novel treatment option for patients with advanced melanoma and is a welcome addition, as we try to better understand tumor immunology. Weber JS, Minor DR, D'Angelo S, et al. ESMO 2014, LBA3_PR
Ipilimumab Plus Sargramostim vs Ipilimumab Alone for Treatment of Metastatic Melanoma – A Randomized Clinical Trial
SUMMARY: It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2) produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints.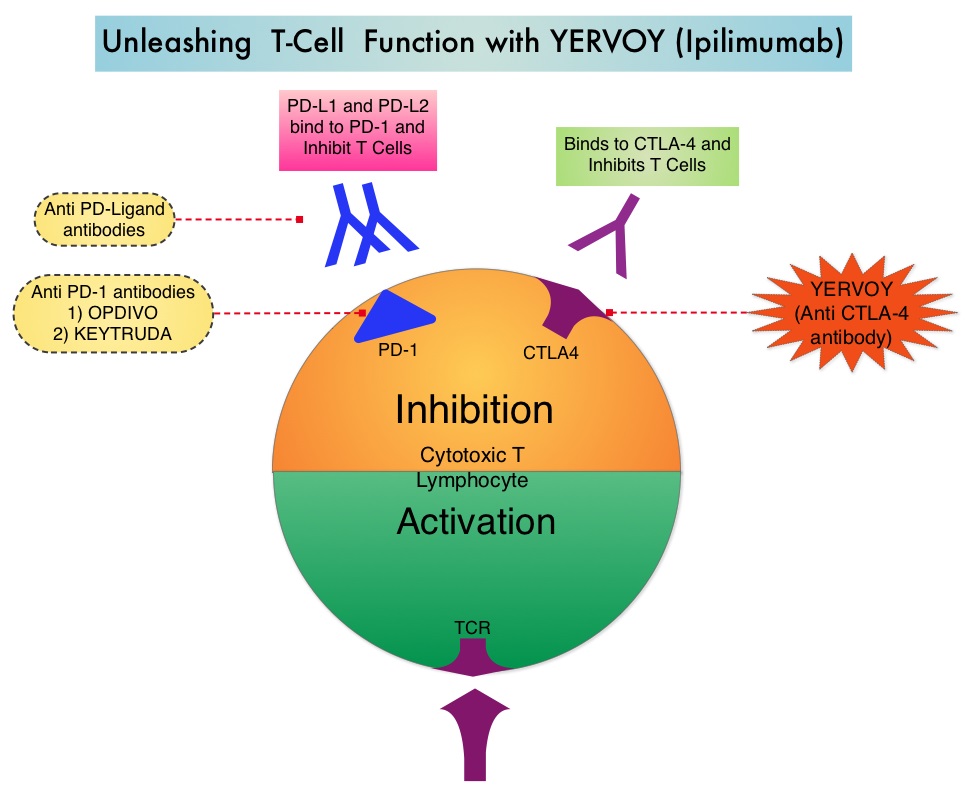 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses. In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753
In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753
The association of indoor tanning and melanoma in adults systematic review and meta-analysis
SUMMARY: It is estimated that in the US, approximately 74,000 new cases of melanoma will be diagnosed and close to 10,000 individuals will die of the disease in 2015. The incidence of melanoma has been on the rise for the past three decades. A major risk factor for most skin cancers is exposure to UltraViolet (UV) radiation, which damages the DNA of skin cells. The main source of UV rays is sunlight, tanning lamps and tanning beds. The 3 main types of UV rays include UVA rays, UVB rays that mainly cause sunburns and UVC rays that do not penetrate through our atmosphere and are not in sunlight. Most indoor tanning beds give off large amounts of UVA rays, which have been found to increase skin cancer risk. It appears that there are no safe UV rays. The International Agency for Research on Cancer has classified Indoor tanning as a Class I carcinogen based on its significant association with malignant melanoma. Indoor tanning with resulting exposure to ultraviolet radiation is a potentially modifiable behavior and several studies to date have shown a relationship between indoor tanning and skin cancer. With this background, the authors reviewed the literature on indoor tanning and gathered data from 31 studies published in peer-reviewed journals that provided risk estimates. These studies included 14,956 cases with malignant melanoma and 233,106 controls. The main focus was to determine the risk of melanoma based on the frequency of use and exposure to the newer indoor tanning beds. They noted that among North Americans, there was a 34% increased risk of developing melanoma in individuals attending more than 10 tanning sessions. Further, those who started indoor tanning before age 25 years, had a 35% risk of developing melanoma and those who ever used indoor tanning were at a 23% increased risk of developing melanoma. It is hypothesized that the newer tanning bed bulb technology, which emits larger doses of long wave UVA rays, has resulted in a 22% increase in the risk of melanoma in individuals who ever used indoor tanning after the year 2000, compared to only 12% in the same population group before the year 2000. The authors concluded that the newer tanning technology is not safer than older techniques and patients should be educated and informed that using tanning beds can be associated with a subsequent diagnosis of malignant melanoma and therefore, cessation of indoor tanning should be strongly encouraged. Colantonio S, Bracken MB, Beecker J. J Am Acad Dermatol. 2014;70:847-857
The International Agency for Research on Cancer has classified Indoor tanning as a Class I carcinogen based on its significant association with malignant melanoma. Indoor tanning with resulting exposure to ultraviolet radiation is a potentially modifiable behavior and several studies to date have shown a relationship between indoor tanning and skin cancer. With this background, the authors reviewed the literature on indoor tanning and gathered data from 31 studies published in peer-reviewed journals that provided risk estimates. These studies included 14,956 cases with malignant melanoma and 233,106 controls. The main focus was to determine the risk of melanoma based on the frequency of use and exposure to the newer indoor tanning beds. They noted that among North Americans, there was a 34% increased risk of developing melanoma in individuals attending more than 10 tanning sessions. Further, those who started indoor tanning before age 25 years, had a 35% risk of developing melanoma and those who ever used indoor tanning were at a 23% increased risk of developing melanoma. It is hypothesized that the newer tanning bed bulb technology, which emits larger doses of long wave UVA rays, has resulted in a 22% increase in the risk of melanoma in individuals who ever used indoor tanning after the year 2000, compared to only 12% in the same population group before the year 2000. The authors concluded that the newer tanning technology is not safer than older techniques and patients should be educated and informed that using tanning beds can be associated with a subsequent diagnosis of malignant melanoma and therefore, cessation of indoor tanning should be strongly encouraged. Colantonio S, Bracken MB, Beecker J. J Am Acad Dermatol. 2014;70:847-857
KEYTRUDA® (Pembrolizumab)
The FDA on September 4, 2014 granted accelerated approval to KEYTRUDA® for the treatment of patients with unresectable or metastatic melanoma and disease progression following YERVOY® (Ipilimumab) and, if BRAF V600 mutation positive, a BRAF inhibitor. KEYTRUDA® is a product of Merck Sharp & Dohme Corp.
KEYTRUDA® – A promising Immunotherapy for Metastatic Melanoma
The FDA granted accelerated approval to KEYTRUDA® (Pembrolizumab), a humanized anti PD-1 antibody, for the treatment of patients with advanced Metastatic Melanoma, who have disease progression following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. KEYTRUDA® produced significant and durable responses in patients with advanced Melanoma, regardless of prior therapy with YERVOY® and this benefit was accomplished with minimal toxicities. This new entry will revolutionize the treatment of advanced Melanoma.
Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL)
SUMMARY: It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2) produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development of various immunotherapies, with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response, by switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or Gate Keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1 (Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma.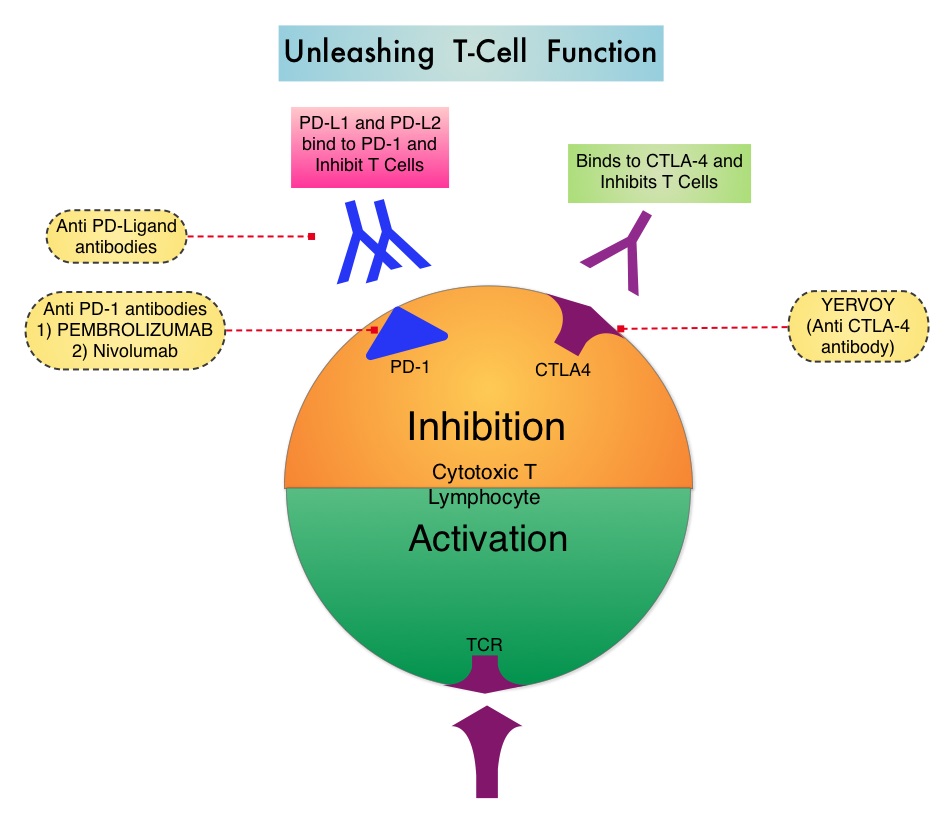 The Food and Drug Administration in May 2014, granted Pembrolizumab a Priority Review designation under its Accelerated Approval Program. Pembrolizumab was previously granted a Breakthrough Therapy designation for advanced melanoma. The authors in this largest phase I clinical trial ever done in patients with malignant melanoma, evaluated the efficacy and safety of Pembrolizumab (formerly known as MK-3475, Lambrolizumab), a humanized monoclonal IgG4 anti PD-1 antibody, in a pooled analysis of 411 patients with advanced melanoma. Of these patients, 221 patients had prior therapy with Ipilimumab (YERVOY® ) and 190 patients were YERVOY® naïve. In this study, three different dosing schedules for Pembrolizumab were utilized – 2 mg/kg every three weeks (N=162), 10 mg/kg every three weeks (N=192) and 10 mg/kg every two weeks (N=57). At the time of this analysis, all patients had at least 6 months of follow up and 75% of the patients had been followed up for at least 9 months. The Overall Response Rate was 40% in the YERVOY® naïve group and 28% in the YERVOY® treated group. Responses were durable and ongoing (88% ongoing) at the time of this analysis. The duration of responses ranged from 6 to 76 weeks, and the median response duration has not yet been reached. The median Progression Free Survival was 24 weeks in YERVOY® naïve group and 23 weeks in the YERVOY® treated group. The median Overall Survival has not been reached at the time of this analysis and the estimated 1 year Overall Survival rate for all patients was 71%. The activity with Pembrolizumab was demonstrated across all dose levels and patient subgroups, irrespective of prior YERVOY® therapy, performance status, LDH levels, BRAF mutation status, tumor stage, and number, as well as type of prior therapies. The most common adverse events of any grade were fatigue, pruritus and rash. Only 4% of the patients discontinued treatment due to a drug related toxicities and overall, 12% of patients experienced grade 3/4 adverse events. The authors concluded that the PD-1 targeting antibody, Pembrolizumab, produced durable responses in patients with advanced melanoma, regardless of prior therapy with YERVOY® and this benefit was accomplished with minimal toxicities. This efficacy data is comparable to another PD-1 targeted monoclonal antibody, Nivolumab. Because of the lack of cross resistance between anti PD-1 antibodies and YERVOY®, combining PD-1 targeted monoclonal antibody with a CTLA-4 targeted antibody such as YERVOY®, could potentially be synergistic, with better outcomes. Ribas A, Hodi FS, Kefford R, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA 9000)</s
The Food and Drug Administration in May 2014, granted Pembrolizumab a Priority Review designation under its Accelerated Approval Program. Pembrolizumab was previously granted a Breakthrough Therapy designation for advanced melanoma. The authors in this largest phase I clinical trial ever done in patients with malignant melanoma, evaluated the efficacy and safety of Pembrolizumab (formerly known as MK-3475, Lambrolizumab), a humanized monoclonal IgG4 anti PD-1 antibody, in a pooled analysis of 411 patients with advanced melanoma. Of these patients, 221 patients had prior therapy with Ipilimumab (YERVOY® ) and 190 patients were YERVOY® naïve. In this study, three different dosing schedules for Pembrolizumab were utilized – 2 mg/kg every three weeks (N=162), 10 mg/kg every three weeks (N=192) and 10 mg/kg every two weeks (N=57). At the time of this analysis, all patients had at least 6 months of follow up and 75% of the patients had been followed up for at least 9 months. The Overall Response Rate was 40% in the YERVOY® naïve group and 28% in the YERVOY® treated group. Responses were durable and ongoing (88% ongoing) at the time of this analysis. The duration of responses ranged from 6 to 76 weeks, and the median response duration has not yet been reached. The median Progression Free Survival was 24 weeks in YERVOY® naïve group and 23 weeks in the YERVOY® treated group. The median Overall Survival has not been reached at the time of this analysis and the estimated 1 year Overall Survival rate for all patients was 71%. The activity with Pembrolizumab was demonstrated across all dose levels and patient subgroups, irrespective of prior YERVOY® therapy, performance status, LDH levels, BRAF mutation status, tumor stage, and number, as well as type of prior therapies. The most common adverse events of any grade were fatigue, pruritus and rash. Only 4% of the patients discontinued treatment due to a drug related toxicities and overall, 12% of patients experienced grade 3/4 adverse events. The authors concluded that the PD-1 targeting antibody, Pembrolizumab, produced durable responses in patients with advanced melanoma, regardless of prior therapy with YERVOY® and this benefit was accomplished with minimal toxicities. This efficacy data is comparable to another PD-1 targeted monoclonal antibody, Nivolumab. Because of the lack of cross resistance between anti PD-1 antibodies and YERVOY®, combining PD-1 targeted monoclonal antibody with a CTLA-4 targeted antibody such as YERVOY®, could potentially be synergistic, with better outcomes. Ribas A, Hodi FS, Kefford R, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA 9000)</s
Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab
SUMMARY: It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease, in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2), produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development of various immunotherapies with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation . The T cells of the immune system play a very important role in modulating the immune system.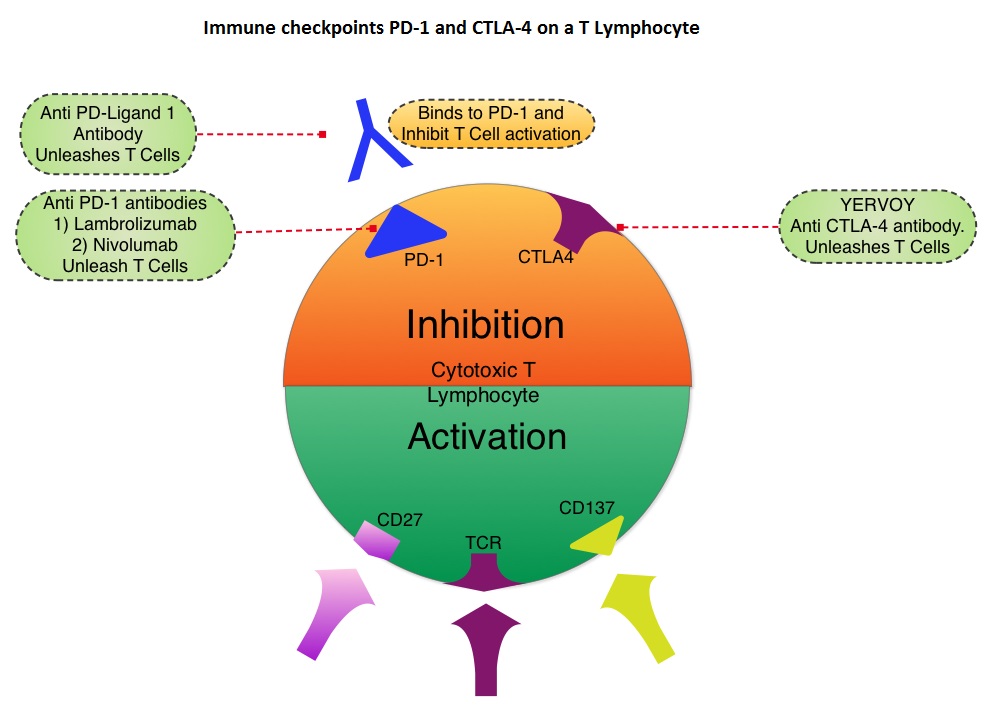 Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. In a previous publication, the authors reported the efficacy results of Nivolumab, a PD-1 targeted, fully human, immunoglobulin G4 monoclonal antibody, which demonstrated an objective response in 20% – 25% of patients with advanced Non Small Cell Lung Cancer, Melanoma and Renal Cell Carcinoma, with favorable toxicities. In this article, the authors reported the outcomes in 107 patients with advanced metastatic melanoma, from the pooled cohort of patients, enrolled between 2008 and 2012. Two thirds of these patients had at least 2 prior treatments, for their advanced disease. These patients received Nivolumab IV, once every 2 weeks, given in an outpatient setting, for up to 96 weeks. Patients were evaluated for Overall Survival, long term safety with treatment and response duration after the treatment was discontinued. The median Overall Survival in those treated with Nivolumab was 16.8 months and 1 and 2-year survival rates were 62% and 43%, respectively. This survival benefit is comparable to that seen following treatment with other agents that are presently available for this patient population, such as YERVOY® (Ipilimumab), ZELBORAF® (Vemurafenib) and combination of BRAF and MEK inhibitors. BRAF mutational status did not impact efficacy of Nivolumab. About 30% of the patients had objective responses and the median response duration was 2 years. The authors hypothesize that the ongoing tumor response following Nivolumab discontinuation, and unlike following chemotherapy, may be due to PD-1 blockade, resulting in the establishment of immune memory response, as is seen after antigen exposure against specific infectious organisms. The most common adverse events of any grade were fatigue, rash and diarrhea. These toxicities were not cumulative. The authors concluded that Nivolumab improved Overall Survival in patients with advanced melanoma and the clinical benefit was durable and persisted even after the drug was discontinued. Studies are underway combining Nivolumab with a different checkpoint inhibitor, YERVOY®. The synergy between these two agents may result in even better outcomes. Topalian SL, Sznol M, McDermott DF, et al. J Clin Oncol 2014;32:1020-1030
Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. In a previous publication, the authors reported the efficacy results of Nivolumab, a PD-1 targeted, fully human, immunoglobulin G4 monoclonal antibody, which demonstrated an objective response in 20% – 25% of patients with advanced Non Small Cell Lung Cancer, Melanoma and Renal Cell Carcinoma, with favorable toxicities. In this article, the authors reported the outcomes in 107 patients with advanced metastatic melanoma, from the pooled cohort of patients, enrolled between 2008 and 2012. Two thirds of these patients had at least 2 prior treatments, for their advanced disease. These patients received Nivolumab IV, once every 2 weeks, given in an outpatient setting, for up to 96 weeks. Patients were evaluated for Overall Survival, long term safety with treatment and response duration after the treatment was discontinued. The median Overall Survival in those treated with Nivolumab was 16.8 months and 1 and 2-year survival rates were 62% and 43%, respectively. This survival benefit is comparable to that seen following treatment with other agents that are presently available for this patient population, such as YERVOY® (Ipilimumab), ZELBORAF® (Vemurafenib) and combination of BRAF and MEK inhibitors. BRAF mutational status did not impact efficacy of Nivolumab. About 30% of the patients had objective responses and the median response duration was 2 years. The authors hypothesize that the ongoing tumor response following Nivolumab discontinuation, and unlike following chemotherapy, may be due to PD-1 blockade, resulting in the establishment of immune memory response, as is seen after antigen exposure against specific infectious organisms. The most common adverse events of any grade were fatigue, rash and diarrhea. These toxicities were not cumulative. The authors concluded that Nivolumab improved Overall Survival in patients with advanced melanoma and the clinical benefit was durable and persisted even after the drug was discontinued. Studies are underway combining Nivolumab with a different checkpoint inhibitor, YERVOY®. The synergy between these two agents may result in even better outcomes. Topalian SL, Sznol M, McDermott DF, et al. J Clin Oncol 2014;32:1020-1030
Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations
SUMMARY:The FDA granted accelerated approval in January 2014, for a combination of MEKINIST® (Trametinib) and TAFINLAR® (Dabrafenib), to treat patients with advanced melanoma, based on the understanding of the biological pathways of this malignancy. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been demonstrated in 6%-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E site and is detected in approximately 50% of melanomas. In the BREAK-3 randomized phase III trial, TAFINLAR®, a selective oral BRAF inhibitor demonstrated a statistically significant improvement in Progression Free Survival (PFS) and Response Rate (RR) compared to Dacarbazine (DTIC) in patients with advanced BRAF V600E mutated melanoma. Squamous cell carcinoma’s were seen in 6% of the patients. In the METRIC phase III study, MEKINIST®, a potent and selective inhibitor of MEK gene (which is downstream from RAF in the MAPK pathway) was compared with either Dacarbazine or TAXOL® (Paclitaxel) in advanced melanoma patients with BRAF V600E/K mutations. Patients in the MEKINIST® group had a significantly improved PFS, RR and Overall Survival. Based on the understanding of the biological pathways of the disease and different mechanisms of action (MOA) of these two agents, a phase I and II trial was conducted combining TAFINLAR® and MEKINIST®. In this study, 162 treatment naïve patients with unresectable or metastatic melanoma, with BRAF V600E or V600K mutations received either TAFINLAR® 150 mg plus MEKINIST® 1 or 2 mg or TAFINLAR® alone. Treatment was given until disease progression or side effects were intolerable. The primary end points were the incidence of Cutaneous Squamous-cell carcinoma, PFS and RR. Secondary end points included Overall Survival and pharmacokinetic activity. The combination treatment resulted in a lower incidence of Cutaneous Squamous-cell carcinoma (7% vs 19% in those receiving monotherapy, P=0.09), improved median PFS (9.4 months vs 5.8 months in the monotherapy group, HR=0.39, P<0.001) and superior complete or partial responses (76% compared with 54% with monotherapy (P=0.03). Pyrexia was however more common in the combination group than in the monotherapy group (71% vs. 26%). The authors concluded that by combining two agents with different MOA’s targeting two different tyrosine kinases in the RAS/RAF/MEK/ERK pathway, PFS was significantly improved. Further, development of resistance can be overcome in BRAF mutation positive melanoma patients and the incidence of secondary skin cancers found with BRAF inhibitor monotherapy can be reduced as well. Flaherty KT, Infante JR, Daud A, et al. N Engl J Med 2012;367:1694-1703
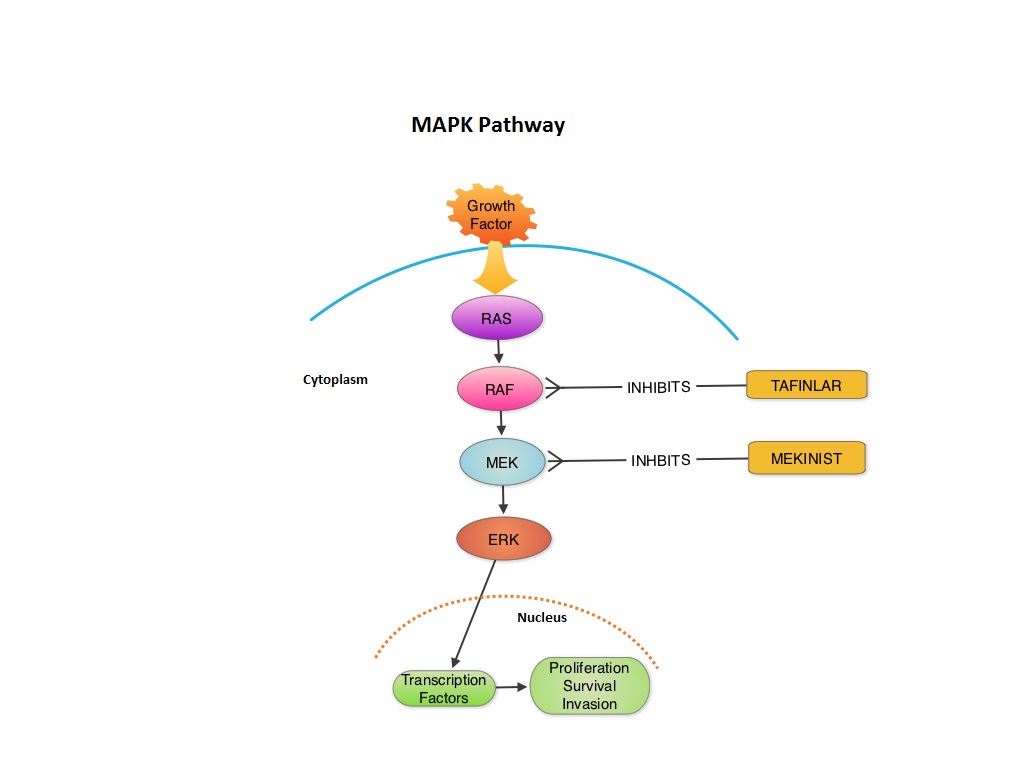
MEKINIST® (Trametinib) in combination with TAFINLAR® (Dabrafenib)
The FDA on January 10, 2014 approved the use of MEKINIST® in combination with TAFINLAR® for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutation, as detected by an FDA-approved test. Both MEKINIST® and TAFINLAR® are products of GlaxoSmithKline, LLC.
