SUMMARY: The U.S. FDA on November 10, 2015, approved COTELLIC® (Cobimetinib) for the treatment of patients with unresectable or metastatic melanoma, with a BRAF V600E or V600K mutation, in combination with ZELBORAF® (Vemurafenib). The American Cancer Society estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway, which enables the cell to respond to external stimuli. This pathway plays a dual role regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas. In the BRIM 3 randomized, phase III study, ZELBORAF® (Vemurafenib), a selective oral inhibitor of mutated BRAF demonstrated significant improvement in Progression Free Survival and Overall Survival compared to Dacarbazine. Squamous cell carcinoma’s were seen in about 6% of the patients treated with BRAF inhibitors. Paradoxical activation of the MAPK pathway in cells without a BRAF mutation has been implicated in the emergence of drug resistance and increased incidence of BRAF-inhibitor induced skin tumors. MEK gene is downstream from RAF in the MAPK pathway. The addition of a selective inhibitor of MEK gene such as COTELLIC® (Cobimetinib) to a BRAF inhibitor such as ZELBORAF®, has addressed some of these limitations, in previously published studies, with improvement in Objective Response rates and decrease in the incidence of cutaneous secondary cancers.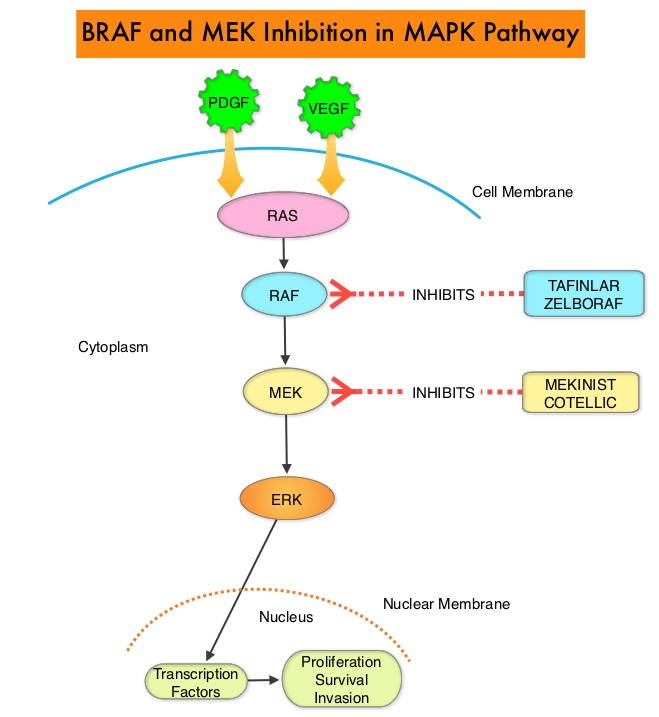
coBRIM is an international, multicenter, randomized, phase III study in which the efficacy and safety of COTELLIC® combined with ZELBORAF®, was evaluated in previously untreated patients, with advanced BRAF-mutated melanoma. Four hundred and ninety five (N=495) patients were randomly assigned in a 1:1 ratio to receive ZELBORAF® 960 mg orally twice daily along with either COTELLIC® 60 mg orally once daily on days 1-21 (N=247) or matching placebo (N=248), of a 28 day cycle. BRAF V600 mutation-positive status was detected using the cobas 4800 BRAF V600 mutation test. The median age of the study group was 55 years and patient demographics in both treatment groups were well balanced. About 60% of the patients, had stage IV disease. The primary endpoint for the study was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Objective Response Rate (ORR), and duration of response.
The primary analysis published in NEJM demonstrated a significant improvement in the median PFS (9.9 months vs 6.2 months) as well as ORR (68% vs 45%) with the combination of ZELBORAF® and COTELLIC® compared to ZELBORAF® and placebo respectively. In this updated analysis submitted to the FDA, the median PFS with the combination of ZELBORAF® and COTELLIC® was 12.3 versus 7.2 months for ZELBORAF® alone (HR= 0.56; P <0.001). There was also a statistically significant improvement in OS based on an interim analysis, with the median OS not reached (NR) in the combination group versus 17 months in the single agent ZELBORAF® group (HR=0.63; P=0.0019). The ORR were 70% and 50% in the ZELBORAF® and COTELLIC® and single agent ZELBORAF® groups, respectively (P<0.001).
The most common adverse reactions were diarrhea, photosensitivity reaction, nausea, pyrexia and vomiting. Treatment-related discontinuation rates in the combination and single agent groups were similar at 13% and 12%, respectively. It was concluded that in patients with unresectable or metastatic melanoma, with a BRAF V600E or V600K mutation, a combination of COTELLIC® and ZELBORAF® delays disease progression and improves survival compared to single agent ZELBORAF®. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: Phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. Larkin JMG, Yan Y, McArthur GA, et al. J Clin Oncol. 2015;33 (suppl; abstr 9006).

