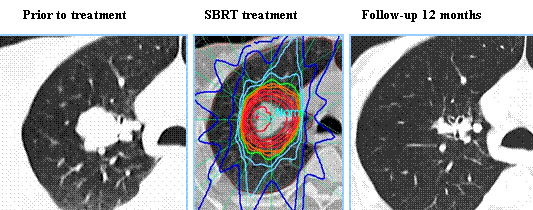
SUMMARY: Lung cancer is the second most common cancer in both men and women and the American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Lobectomy is the treatment of choice for resectable Non Small Cell Lung Cancer (NSCLC). Pneumonectomy is rarely performed due to unacceptably high mortality rate. Sublobar resection (Wedge resection or Segmentectomy) is considered a “compromise operation” in selected high risk patients with early stage lung cancer. With the approval of lung cancer screening in high risk individuals and subsequent detection of small tumors, Sublobar resections have been on the rise, even in good-risk patients, in many institutions. Sublobar resection includes Wedge resection and Segmentectomy. In Wedge resection, the lung tumor is removed with a surrounding margin of normal lung tissue, and is not an anatomical resection. Segmentectomy, unlike Wedge resection, is an anatomical resection that usually includes one or more pulmonary parenchymal segments with the dissection of intraparenchymal and hilar lymph nodes. Wedge resection is inferior to anatomic Segmentectomy and is associated with an increased risk of local recurrence and decreased survival in patients with Stage I NSCLC.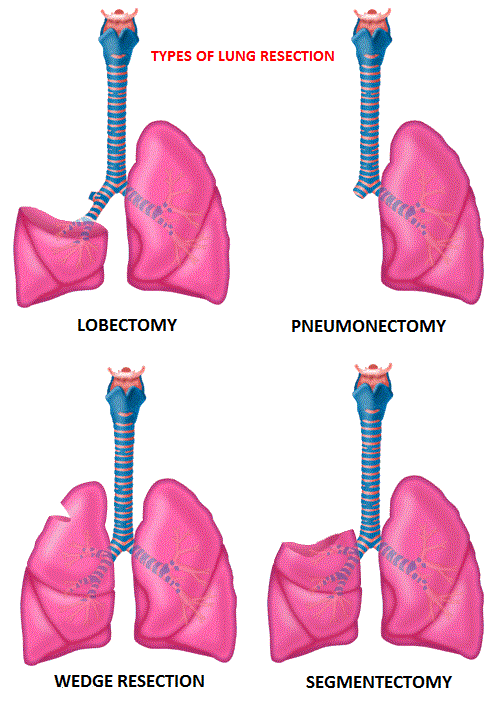
The authors in this study analyzed the National Cancer Data Base (NCDB) and the primary goal of this study was to understand practice patterns in the surgical management of patients with clinical Stage IA NSCLC, as well as identify predictors of surgical management with Sublobar resection versus Lobectomy and also evaluate the extent of pathologic lymph node assessment, performed in association with Sublobar resections, in a community practice setting. A secondary goal was to compare long term survival between Sublobar resection versus Lobectomy.
In this large analysis, 39,403 patients from the National Cancer Data Base (NCDB) were included, of whom 75.5% (N=29,736) underwent Lobectomy and 24.5% (N=9667) had Sublobar resection (Wedge resection 84.7%; N = 8192 and Segmental resection 15.3%; N = 1475). Lymph node evaluation was not performed in 2788 (28.8%) of Sublobar resection patients, and 7298 (75.5%) of Sublobar resections were for tumors ≤ 2 cm.
It was noted that Lobectomy was associated with significantly improved 5-year survival compared to Sublobar resection (66.2% vs. 51.2%; adjusted HR=0.66; P <0 .001). Among patients who underwent Sublobar resection, lymph node sampling was associated with significantly better 5-year survival compared to patients who did not have lymph node sampling (58.2% vs. 46.4%; P < 0.001), although these outcomes were still inferior to Lobectomy.
The authors concluded that for patients with Stage 1A NSCLC, surgical Lobectomy significantly improved survival compared to Sublobar resection. Patients ineligible for Lobectomy and treated with Sublobar resection, should undergo lymph node samplings to help guide appropriate post operative therapy. Sublobar Resection for Clinical Stage IA Non–small-cell Lung Cancer in the United States. Speicher PJ, Gu L, Gulack BC, et al. Clinical Lung Cancer 2016;17:47-55

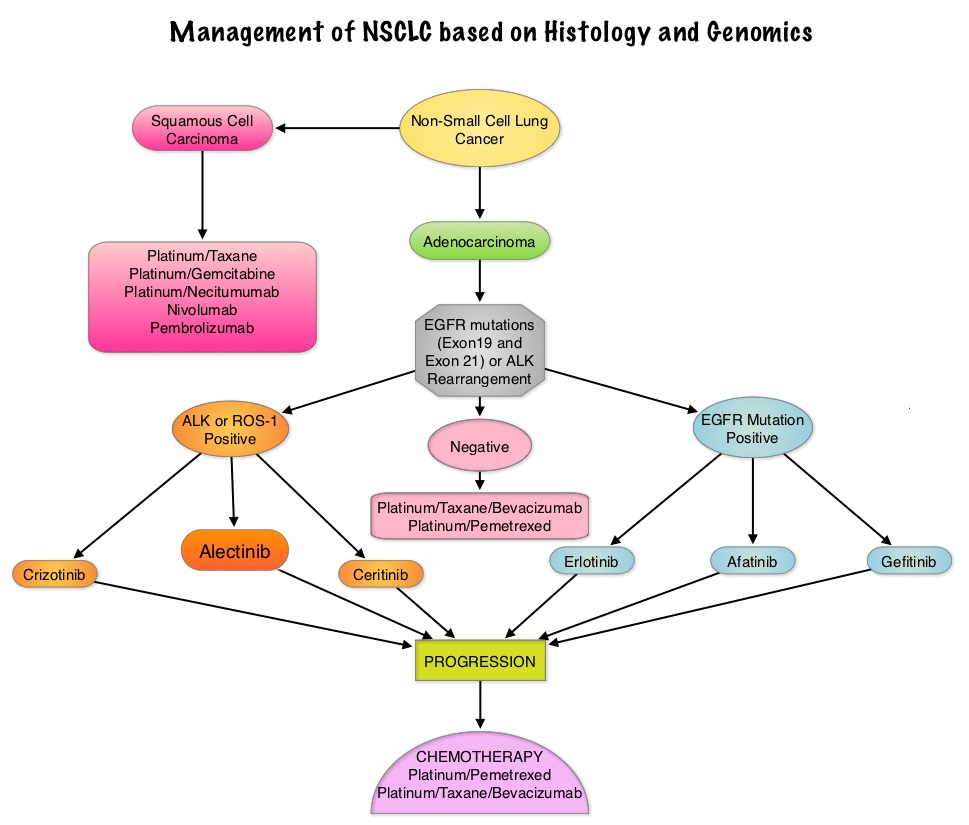
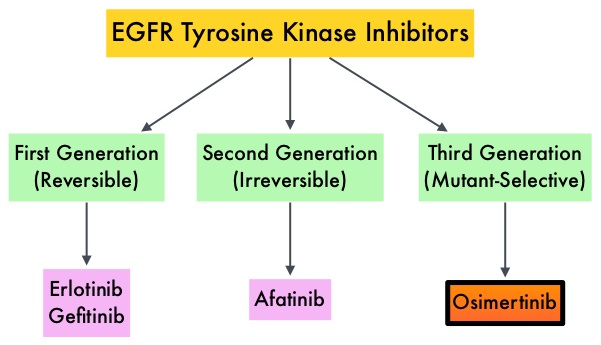
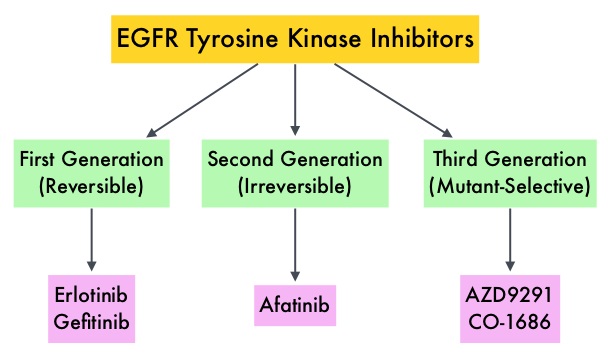
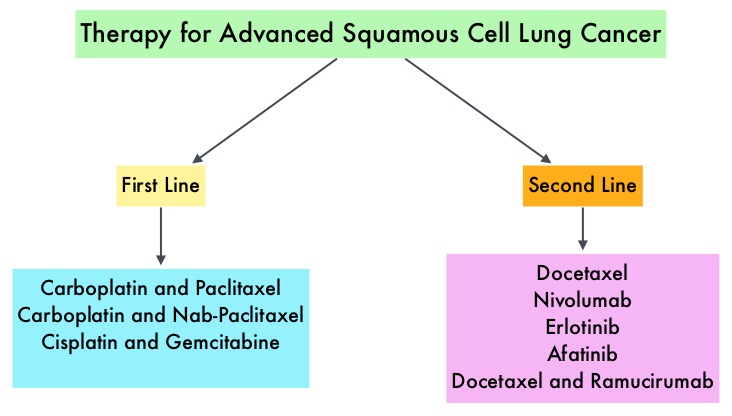 Non Small Cell Lung Cancer patients with squamous cell histology have been a traditionally hard- to-treat patient group, with less than 5% of patients with advanced SCC, surviving for five years or longer. Some of the advanced NSCLC tumors are dependent on the Epidermal Growth Factor Receptor (EGFR) for cell proliferation and survival, regardless of EGFR mutation status. TARCEVA® (Erlotinib) is a reversible EGFR Tyrosine Kinase Inhibitor and is presently approved by the FDA for the treatment of locally advanced or metastatic NSCLC, after failure of at least one prior chemotherapy regimen. GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. This kinase inhibitor is indicated for the first line treatment of patients with metastatic NSCLC, whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
Non Small Cell Lung Cancer patients with squamous cell histology have been a traditionally hard- to-treat patient group, with less than 5% of patients with advanced SCC, surviving for five years or longer. Some of the advanced NSCLC tumors are dependent on the Epidermal Growth Factor Receptor (EGFR) for cell proliferation and survival, regardless of EGFR mutation status. TARCEVA® (Erlotinib) is a reversible EGFR Tyrosine Kinase Inhibitor and is presently approved by the FDA for the treatment of locally advanced or metastatic NSCLC, after failure of at least one prior chemotherapy regimen. GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. This kinase inhibitor is indicated for the first line treatment of patients with metastatic NSCLC, whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.