SUMMARY: The FDA granted accelerated approval to KEYTRUDA® (Pembrolizumab), for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors express Programmed Death Ligand 1 (PD-L1), as determined by an FDA-approved test, following disease progression on or after platinum-containing chemotherapy. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers.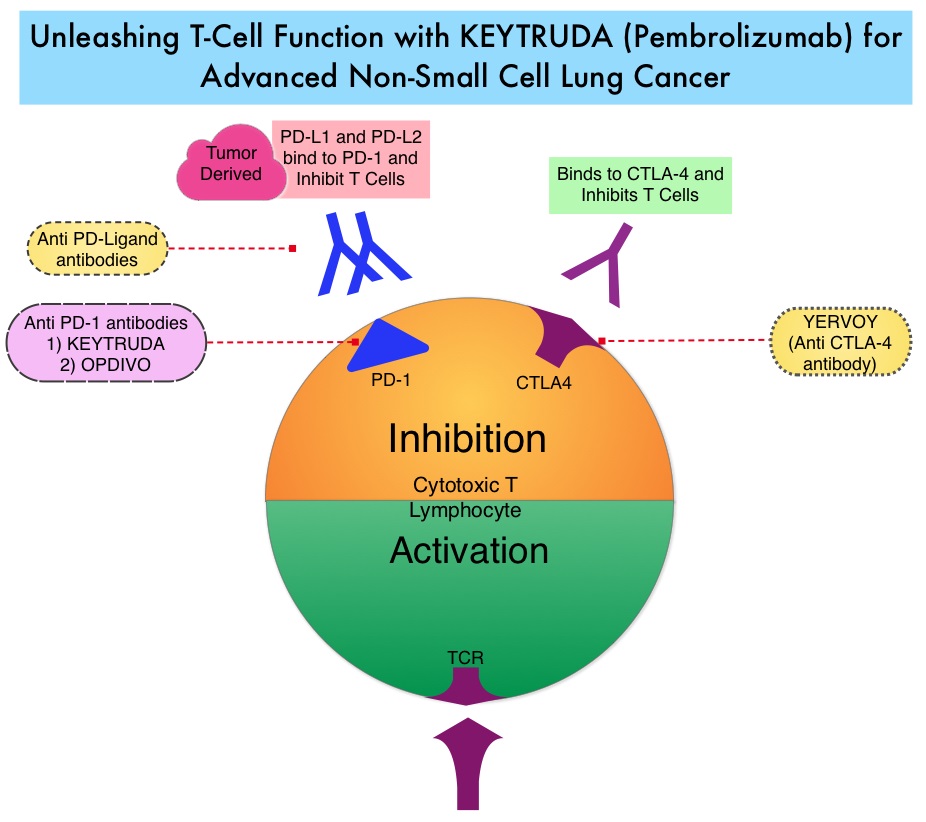
The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers, switch off the T cells of the immune system and thereby inhibit an intense immune response. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed, that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. Targeting Immune checkpoints unleashes the T cells, resulting in T cell proliferation, activation and a therapeutic response. KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells.
In this publication, the authors assessed the efficacy and safety of KEYTRUDA® in patients with advanced NSCLC enrolled in the KEYNOTE-001 phase I trial. Four Hundred and Ninety five (N=495) patients were assigned to either a training group (N=182) or a validation group (N=313) and KEYTRUDA® was administered at three dosages: 2 mg/kg IV every 3 weeks, 10 mg/kg IV every 3 weeks, or 10 mg/kg IV every 2 weeks. Patient responses were assessed every 9 weeks.
The Objective Response Rate (ORR) in the entire study population was 19.4%, the median Duration of Response was 12.5 months, the median Progression Free Survival was 3.7 months and the median Overall Survival was 12.0 months. The PD-L1 (Programmed Death-Ligand 1) expression was evaluated in 204 patients in the validation group by ImmunoHisto Chemistry (IHC) and membrane PD-L1 expression of 50% or more, in tumor cells, was selected as the cutoff. It was noted that among patients with PD-L1 expression in at least 50% of tumor cells, the Objective Response Rate was 45.2%, median Progression Free Survival was 6.3 months and median Overall Survival has not been reached. Responses were not as robust in those patients with tumors demonstrating less than 50% PD-L1 expression, but in those who did respond, the duration of responses were comparable to those with 50% or more PD-L1 expression. KEYTRUDA® was well tolerated overall and the common immune mediated adverse events were infusion reactions, hypothyroidism and pneumonitis.
The authors concluded that KEYTRUDA® showed significant antitumor activity in patients with advanced Non Small Cell Lung Cancer, whose tumor PD-L1expression was 50% or more. Further, the median duration of response exceeded 12 months among responders, regardless of the degree of PD-L1 expression. This study validated that PD-L1 expression in tumors is clearly a marker of response to KEYTRUDA®. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. Garon EB, Rizvi NA, Hui R, et al. N Engl J Med 2015; 372:2018-2028

