The FDA on July 13, 2015 approved IRESSA® for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA approved test. IRESSA® was approved concurrently with a labeling expansion of the therascreen EGFR RGQ PCR Kit, a companion diagnostic test, for patient selection. IRESSA® tablets are a product of AstraZeneca Pharmaceuticals LP.
Tag: Lung Cancer: Non-Small Cell
Late Breaking Abstract – ASCO 2015 OPDIVO® Improves Overall Survival in Non- Squamous NSCLC Patients
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 25% are Squamous cell carcinomas, 40% are Adenocarcinomas and 10% are Large cell carcinomas. The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system.  Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s
Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab) , an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The U. S. Food and Drug Administration granted approval to OPDIVO®, for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC), with progression on or after platinum based chemotherapy. CheckMate 057 is a randomized, international, phase 3 study designed to evaluate the benefit of OPDIVO® for patients with Non-Squamous (NSQ) NSCLC who had progressed after platinum-based doublet chemotherapy. A total of 582 patients were randomized to receive OPDIVO® 3 mg/kg IV every 2 weeks (n=292) or TAXOTERE® 75 mg/m2 IV every 3 weeks (n=290). Eligible patients included those with advanced Non-Squamous NSCLC who had progressed after platinum-based doublet chemotherapy and a Tyrosine Kinase Inhibitor (TKI), if deemed eligible for a TKI. Treatment was continued until disease progression or unacceptable toxicity. The primary clinical endpoint was Overall Survival (OS). Secondary endpoints included Objective Response Rate (ORR), Progression Free Survival (PFS), Efficacy based on PD-L1 expression, Quality of Life, and Safety. The study was stopped earlier than expected following assessment by the independent Data Monitoring Committee (DMC) which concluded that the study met its endpoint, demonstrating superior overall survival, in patients receiving OPDIVO®, compared to the control group. Patients in the OPDIVO®, group had a significantly higher median OS compared to those in the TAXOTERE® group (12.2 months versus 9.4 months, Hazard Ratio [HR] 0.73, P=0.0015). This meant a 27% reduction in the risk of death in the OPDIVO® group and this survival benefit was seen in all predefined subgroup of patients. The Objective Response Rate (ORR) was also significantly higher for patients receiving OPDIVO® compared to TAXOTERE® (19% versus 12%, P=0.0246) and the median duration of response (DOR) was significantly higher for the OPDIVO® group (17.2 months) vs the TAXOTERE® group (5.6 months). More importantly, when tumor PD-L1 expression was correlated with Overall Survival, the median OS for OPDIVO® was 17.2 months, 18.2 months, and 19.4 months for patients with tumors having 1% or higher, 5% or higher, and 10% or higher of cells staining positive for PD-L1, respectively, compared with 9.0 months, 8.1 months, and 8.0 months with TAXOTERE® treatment. Even though this study showed significant survival outcomes for patients expressing any level of PD-L1, the magnitude of benefit was even more so, in patients with tumors expressing higher levels of PD-L1. PD-L1 expression may therefore be a predictor of response, although this should not yet be used for patient selection. Grade 3-5 adverse events occurred more often in the TAXOTERE® group compared to the OPDIVO® group (54% vs 10%). Based on this compelling data, the authors concluded that OPDIVO® significantly improves Overall Survival when compared to TAXOTERE®, in patients with advanced non-Squamous NSCLC, after failure of platinum based doublet therapy. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). Paz-Ares L, Horn L, Borghaei H, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA109)</s
Targeting ROS1 Molecular Driver Mutations with XALKORI® in NSCLC
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 25% are Squamous cell carcinomas, 40% are Adenocarcinomas and 10% are Large cell carcinomas. There is now growing body of evidence suggesting superior outcomes when advanced NSCLC patients with specific genomic alterations receive targeted therapies.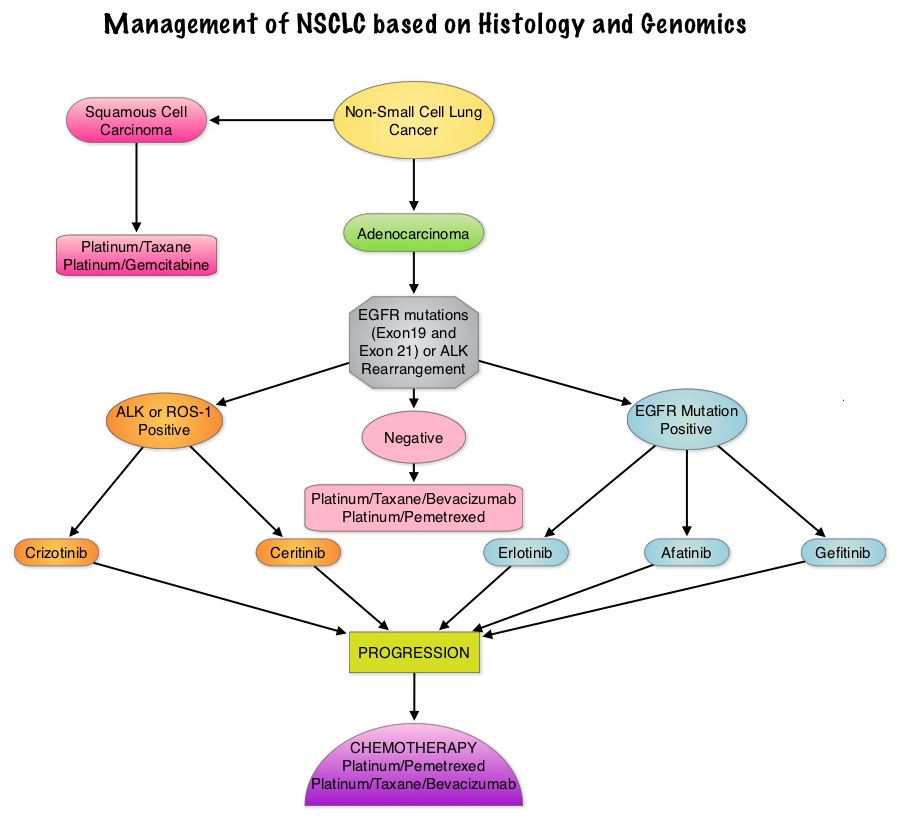 Approximately 1% – 2% of lung adenocarcinomas harbor ROS1 gene rearrangements. ROS1 gene is located on chromosome 6q22 (long arm of chromosome 6) and plays an important role in cell growth and development. ROS1 gene fusion with another gene results in a mutated DNA sequence which then produces an abnormal protein responsible for unregulated cell growth and cancer. ROS1 gene rearrangement has been identified as a driver mutation in Non Small cell Lung Cancer with adenocarcinoma histology. This is more common in nonsmokers or in light smokers (<10 pack years) who are relatively young (average age of 50 years) and thus share similar characteristics with ALK-positive patients. The ROS protein and the ALK protein have similar structure and function and are sensitive to Tyrosine Kinase Inhibitors such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib). ROS1 mutations have been also been associated with Cholangiocarcinoma (Bile duct cancer) and Glioblastoma multiforme. ROS1 rearrangements are mutually exclusive with other oncogenic mutations found in NSCLC such as EGFR mutations, KRAS mutations and ALK rearrangement. The presence of a ROS1 rearrangement can be detected by Fluorescence In Situ Hybridization (FISH), ImmunoHistoChemistry (IHC), Reverse Transcriptase– Polymerase Chain Reaction (RT-PCR) and Next Generation-Sequencing. XALKORI® is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In a previously published expansion cohort of the phase 1 study by Shaw and colleagues ( NEJM 2014; 371:1963-1971), XALKORI® showed significant activity in patients with in patients with advanced ROS1rearranged NSCLC. The authors in this publication provided additional evidence that ROS1 gene rearrangement is an actionable target in NSCLC, by conducting a retrospective study in centers that tested for ROS1 rearrangement and evaluated the outcomes of ROS1-positive NSCLC patients, who had been treated with XALKORI®. They included 32 patients with NSCLC whose tumors showed ROS1 rearrangement and who had received off-label treatment with XALKORI®. The median age was 50.5 years. They noted an overall response rate of 80% and a disease control rate, 86.7%. The median Progression Free Survival (PFS) was 9.1 months, and the PFS rate at 12 months was 44%. This impressive efficacy data again validates that similar to EGFR mutations and ALK rearrangements, ROS1 gene rearrangements are molecular drivers and patients with NSCLC with adenocarcinoma histology should be tested for ROS1. Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results From the EUROS1 Cohort. Mazières J, Zalcman G, Crinò L, J Clin Oncol. 2015;33:992-999
Approximately 1% – 2% of lung adenocarcinomas harbor ROS1 gene rearrangements. ROS1 gene is located on chromosome 6q22 (long arm of chromosome 6) and plays an important role in cell growth and development. ROS1 gene fusion with another gene results in a mutated DNA sequence which then produces an abnormal protein responsible for unregulated cell growth and cancer. ROS1 gene rearrangement has been identified as a driver mutation in Non Small cell Lung Cancer with adenocarcinoma histology. This is more common in nonsmokers or in light smokers (<10 pack years) who are relatively young (average age of 50 years) and thus share similar characteristics with ALK-positive patients. The ROS protein and the ALK protein have similar structure and function and are sensitive to Tyrosine Kinase Inhibitors such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib). ROS1 mutations have been also been associated with Cholangiocarcinoma (Bile duct cancer) and Glioblastoma multiforme. ROS1 rearrangements are mutually exclusive with other oncogenic mutations found in NSCLC such as EGFR mutations, KRAS mutations and ALK rearrangement. The presence of a ROS1 rearrangement can be detected by Fluorescence In Situ Hybridization (FISH), ImmunoHistoChemistry (IHC), Reverse Transcriptase– Polymerase Chain Reaction (RT-PCR) and Next Generation-Sequencing. XALKORI® is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In a previously published expansion cohort of the phase 1 study by Shaw and colleagues ( NEJM 2014; 371:1963-1971), XALKORI® showed significant activity in patients with in patients with advanced ROS1rearranged NSCLC. The authors in this publication provided additional evidence that ROS1 gene rearrangement is an actionable target in NSCLC, by conducting a retrospective study in centers that tested for ROS1 rearrangement and evaluated the outcomes of ROS1-positive NSCLC patients, who had been treated with XALKORI®. They included 32 patients with NSCLC whose tumors showed ROS1 rearrangement and who had received off-label treatment with XALKORI®. The median age was 50.5 years. They noted an overall response rate of 80% and a disease control rate, 86.7%. The median Progression Free Survival (PFS) was 9.1 months, and the PFS rate at 12 months was 44%. This impressive efficacy data again validates that similar to EGFR mutations and ALK rearrangements, ROS1 gene rearrangements are molecular drivers and patients with NSCLC with adenocarcinoma histology should be tested for ROS1. Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results From the EUROS1 Cohort. Mazières J, Zalcman G, Crinò L, J Clin Oncol. 2015;33:992-999
Post Operative Radiation Therapy (PORT) Improves Survival in Resected N2 Non-Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Based on the extent of the disease and the treatment approach, patients with localized or locally advanced NSCLC can be divided into two groups – 1) Surgically resectable disease group (stage I, stage II, and selected stage III tumors) for whom postoperative Cisplatin-based combination chemotherapy may provide a survival advantage (particularly for those with resected stage II or stage IIIA NSCLC). 2) Locally (T3–T4) and/or regionally (N2–N3) advanced disease group who have unresectable disease, who benefit with radiation therapy in combination with chemotherapy. Based on available evidence, postoperative chemotherapy is not recommended outside of a clinical trial for patients with completely resected stage I NSCLC. Although there is sufficient evidence for postoperative chemotherapy in patients with stage II or stage IIIA NSCLC, its usefulness in patients with stage IB NSCLC remains unclear. The value of adjuvant Post Operative Radiation Therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC. The risk of locoregional recurrence (LRR) is 20-40% in patients with resected node-positive disease and this in turn may independently contribute to worsened Overall Survival in patients with NSCLC. Nonetheless, a large meta-analysis from trials conducted mainly in the 1960’s and 1970’s showed that adjuvant Post Operative Radiation Therapy (PORT) in stages IIA NSCLC and IIB NSCLC was associated with an 18% relative increase in the risk of death compared with surgery alone. This decrease in Overall Survival has been attributed to outmoded RT techniques and doses resulting in cardiac and pulmonary toxicity. This study was conducted to evaluate the impact of modern (Computed Tomography simulation and at least Linear accelerator- Linac based, three-dimensional, conformal RT) Post Operative Radiation Therapy (PORT) on Overall Survival (OS) in a large population-based registry of patients with completely resected stage IIIA (N2) NSCLC, when compared with adjuvant chemotherapy alone. The authors identified 4,483 patients in the National Cancer Data Base from 2006 to 2010 with pathologic N2, NSCLC, who underwent complete resection and adjuvant chemotherapy. This large patient population was representative of typical patients treated throughout the United States. Of these large cohort of patients, 1,850 had received PORT (45 Gy or more) and 2,633 patients did not. The authors evaluated the impact of patient and treatment variables on OS. The median follow-up time was 22 months. The use of PORT was associated with an increase in median and 5-year OS compared with no PORT (median OS, 45.2 vs 40.7 months, respectively; 5-year OS, 39.3% vs 34.8% respectively; P= 0.014). The improved OS remained, independently predicted by younger age, female sex, urban population, fewer comorbidities, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT. The authors concluded that modern Post Operative Radiotherapy Therapy confers an additional OS advantage beyond that achieved with adjuvant chemotherapy alone, for patients with N2, NSCLC after complete resection and adjuvant chemotherapy. Postoperative Radiotherapy for Pathologic N2 Non–Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: A Review of the National Cancer Data Base. Robinson CG, Patel AP, Bradley JD, et al. J Clin Oncol. 2015; 33:870-876
Although there is sufficient evidence for postoperative chemotherapy in patients with stage II or stage IIIA NSCLC, its usefulness in patients with stage IB NSCLC remains unclear. The value of adjuvant Post Operative Radiation Therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC. The risk of locoregional recurrence (LRR) is 20-40% in patients with resected node-positive disease and this in turn may independently contribute to worsened Overall Survival in patients with NSCLC. Nonetheless, a large meta-analysis from trials conducted mainly in the 1960’s and 1970’s showed that adjuvant Post Operative Radiation Therapy (PORT) in stages IIA NSCLC and IIB NSCLC was associated with an 18% relative increase in the risk of death compared with surgery alone. This decrease in Overall Survival has been attributed to outmoded RT techniques and doses resulting in cardiac and pulmonary toxicity. This study was conducted to evaluate the impact of modern (Computed Tomography simulation and at least Linear accelerator- Linac based, three-dimensional, conformal RT) Post Operative Radiation Therapy (PORT) on Overall Survival (OS) in a large population-based registry of patients with completely resected stage IIIA (N2) NSCLC, when compared with adjuvant chemotherapy alone. The authors identified 4,483 patients in the National Cancer Data Base from 2006 to 2010 with pathologic N2, NSCLC, who underwent complete resection and adjuvant chemotherapy. This large patient population was representative of typical patients treated throughout the United States. Of these large cohort of patients, 1,850 had received PORT (45 Gy or more) and 2,633 patients did not. The authors evaluated the impact of patient and treatment variables on OS. The median follow-up time was 22 months. The use of PORT was associated with an increase in median and 5-year OS compared with no PORT (median OS, 45.2 vs 40.7 months, respectively; 5-year OS, 39.3% vs 34.8% respectively; P= 0.014). The improved OS remained, independently predicted by younger age, female sex, urban population, fewer comorbidities, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT. The authors concluded that modern Post Operative Radiotherapy Therapy confers an additional OS advantage beyond that achieved with adjuvant chemotherapy alone, for patients with N2, NSCLC after complete resection and adjuvant chemotherapy. Postoperative Radiotherapy for Pathologic N2 Non–Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: A Review of the National Cancer Data Base. Robinson CG, Patel AP, Bradley JD, et al. J Clin Oncol. 2015; 33:870-876
OPDIVO® now Approved for Advanced, Refractory Squamous Non-Small Cell Lung Cancer
SUMMARY: The U. S. Food and Drug Administration granted approval to Nivolumab (OPDIVO®) for the treatment of patients with metastatic squamous Non-Small Cell Lung Cancer (NSCLC) with progression on or after platinum based chemotherapy. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and a over 158,000 patients will die of the disease. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 25% are Squamous cell carcinomas, 40% are Adenocarcinomas and 10% are Large cell carcinomas. Non-Small Cell Lung Cancer patients with Squamous cell histology have been a traditionally hard- to-treat patient group. 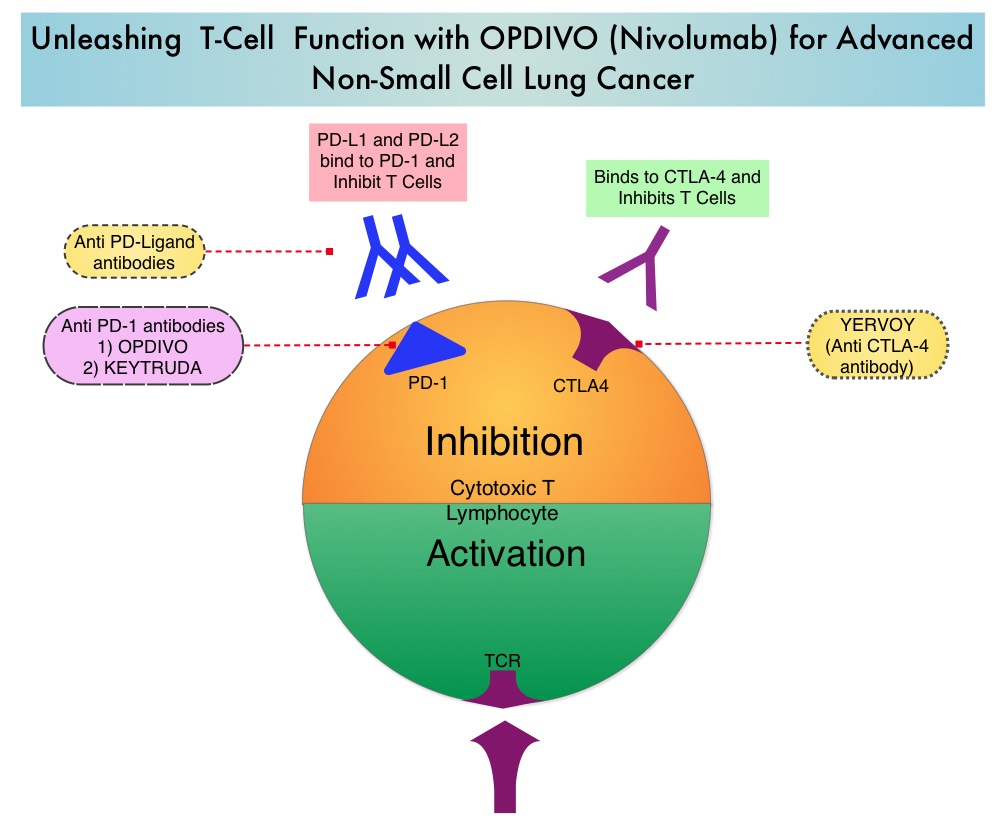 The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
OPDIVO® (Nivolumab)
The FDA on March 4, 2015 granted approval to OPDIVO® for the treatment of patients with metastatic Squamous Non-Small Cell Lung Cancer (NSCLC) with progression on or after platinum-based chemotherapy. OPDIVO® was previously approved in December, 2014 for the treatment of patients with unresectable or metastatic melanoma and disease progression following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. OPDIVO® is a product of Bristol-Myers Squibb Company.
CYRAMZA® (Ramucirumab)
The FDA on December 12, 2014 approved CYRAMZA® for use in combination with TAXOTERE® (Docetaxel) for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC) with disease progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving CYRAMZA®. CYRAMZA® injection for intravenous infusion is a product of Eli Lilly and Company.
Molecular Testing for Selection of Patients with Lung Cancer for Epidermal Growth Factor Receptor and Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitors American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Guideline
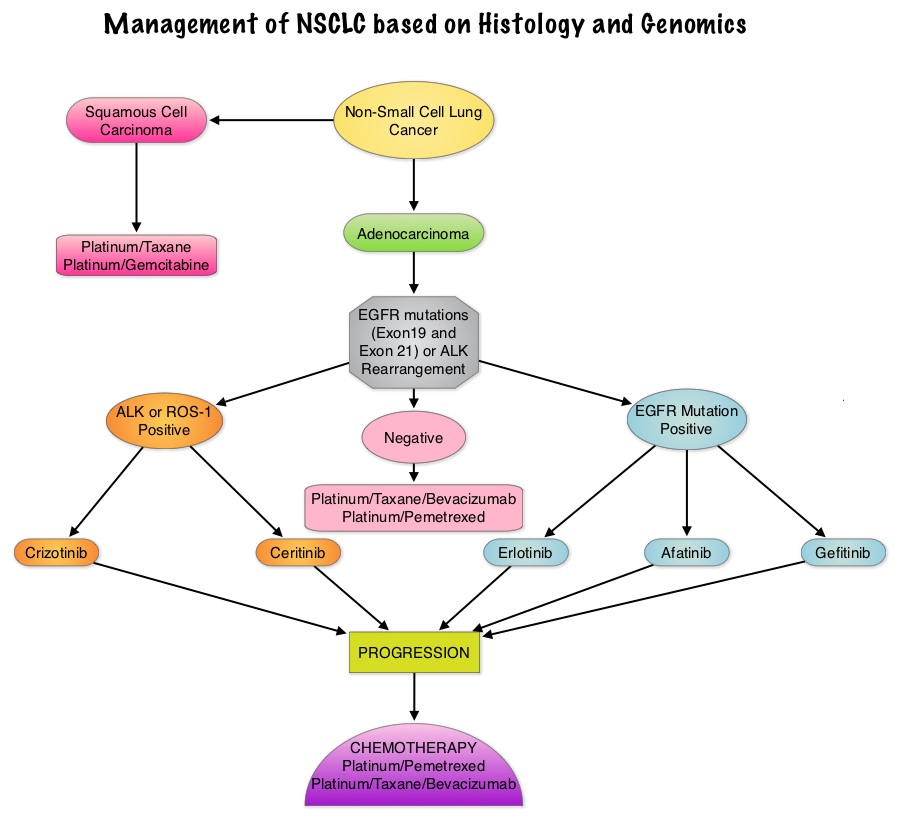
SUMMARY: There is now growing body of evidence suggesting superior outcomes when advanced NSCLC patients with specific genomic alterations receive targeted therapies. Following review of 127 studies by experts and input from a scientific advisory panel, The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) offered evidence-based recommendations for the molecular analysis of lung cancers for Epidermal Growth Factor Receptor (EGFR ) mutations and Anaplastic Lymphoma Kinase (ALK) rearrangements, thereby selecting patients with lung cancer, for treatment with EGFR and ALK tyrosine kinase inhibitors. The ASCO review panel has endorsed these guidelines which specifically address the following questions:
1) Which patients should be tested for EGFR mutations and ALK rearrangements?
EGFR or ALK testing is recommended for all patients with advanced lung adenocarcinoma or tumors with an adenocarcinoma component, irrespective of clinical characteristics such as smoking history, sex, race, or other clinical factors. Tumor samples of other histologies for which an adenocarcinoma component cannot be excluded because of sampling, can be considered for testing, particularly if clinical criteria are suggestive (eg, younger age, lack of smoking history). Both primary tumors and metastatic lesions are suitable for testing. When fully excised lung cancer specimens are available, EGFR and ALK testing is not recommended in lung cancers that lack any adenocarcinoma component, such as pure squamous cell carcinomas, pure small-cell carcinomas, or large-cell carcinomas lacking IHC (ImmunoHistoChemistry) evidence of adenocarcinoma differentiation.
2) When should a patient specimen be tested for EGFR mutation or ALK rearrangement?
Testing should be ordered at the time of diagnosis of advanced disease or recurrence. For patients with earlier stage disease who undergo surgical resection, testing at the time of diagnosis is encouraged so that molecular information is available to an oncologist at the time of recurrence, for a subset of patients who subsequently experience recurrence. Tissue should be prioritized for EGFR and ALK testing.
 3) How rapidly should test results be available?
3) How rapidly should test results be available?
Laboratory turnaround times of 5 to 10 working days (2 weeks) for EGFR and ALK results are recommended.
4) How should specimens be processed for EGFR mutation testing?
Pathologists should use Formalin-Fixed, Paraffin-Embedded (FFPE) specimens or fresh frozen or alcohol-fixed specimens for PCR based EGFR mutation tests. EGFR and ALK testing can be performed with cytology samples, with cell blocks being preferred over smear preparations.
5) How should EGFR testing be performed?
EGFR testing should detect mutations in samples composed of as few as 50% tumor cells, although sensitivity to detect mutations in samples containing > 10% tumor cells is strongly encouraged. Sensitizing EGFR mutations with a population frequency of at least 1% should be reported. IHC for total EGFR as well as EGFR copy number analysis by FISH (Fluorescence In Situ Hybridization) is not recommended.
6) What is the role of KRAS analysis in selecting patients for targeted therapy with EGFR TKIs?
KRAS mutations are common (30%) in lung adenocarcinomas and mutually exclusive with EGFR and ALK. Testing for KRAS may be performed initially to exclude KRAS mutated tumors from EGFR and ALK testing but KRAS mutation testing is not recommended as a sole determinant of EGFR-targeted therapy.
7) What additional testing considerations are important in the setting of secondary or acquired EGFR TKI resistance?
If a laboratory performs testing on specimens from patients with acquired resistance to EGFR kinase inhibitors, such tests should be able to detect the secondary EGFR T790M mutation in as few as 5% of cells.
8) What methods should be used for ALK testing?
ALK FISH assay using dual labeled break-apart probes should be used for selecting patients for ALK TKI therapy. ALK IHC, if carefully validated, may be considered as a screening methodology to select specimens for ALK FISH testing. RT-PCR (Reverse Transcription–Polymerase Chain Reaction) is not recommended as an alternative to FISH, for selecting patients for ALK inhibitor therapy.
9) Are other molecular markers suitable for testing in lung cancer?
Testing for EGFR should be prioritized over other molecular markers in lung adenocarcinoma followed by testing for ALK. Testing for ROS1 and RET rearrangements may soon become a part of the guidelines.
10) How should molecular testing of lung adenocarcinomas be implemented and operationalized?
Pathology departments should establish a process wherein tissue (blocks or unstained slides) is sent to outside molecular laboratories within 3 days of receiving a request and to in house molecular laboratories within 24 hours. Results should be available within 2 weeks and reported in a format that is easily understood by oncologists and nonspecialist pathologists.
Leighl NB, Rekhtman N, Biermann WA, et al. J Clin Oncol 2014;32:3673-3679
Screening for Lung Cancer US Preventive Services Task Force Recommendation Statement
SUMMARY: The Centers for Medicare & Medicaid Services (CMS) on November 14, 2014, proposed that the evidence is sufficient, to add a Lung cancer screening counseling and shared decision making visit for appropriate beneficiaries. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. Given the incidence and mortality related to Lung cancer, several studies were conducted dating back to the 1960’s and 1970’s in an attempt to find an appropriate screening test for Lung cancer. They included testing sputum cytology and chest radiography or a combination of both. However, these screening methodologies did not conclusively demonstrate improvements in health outcomes. The results of a NCI-sponsored National Lung Screening Trial (NLST) published in 2011, was more optimistic. In this federally funded U.S. study, 53,439 asymptomatic participants, 55 to 74 years of age, with at least 30 pack-year smoking history were enrolled and randomized to undergo annual screening with either Low dose CT scan (n=26,715) or a chest X-Ray (n=26,724), for three years. The use of Low Dose CT (LDCT) scans for 3 years in this high risk, healthy patients, resulted in a 20% reduction in Lung cancer mortality, compared to screening with a chest X-Ray. Based on these findings, Lung cancer screening was felt appropriate for the following groups of patients:
1) People 55-74 years of age with no signs or symptoms of Lung disease or lung Cancer
2) Current or former smoker with a 30 pack year smoking history (Number of years smoked multiplied by the number of packs of cigarettes per day with each pack containing 20 cigarettes)
3) Former smokers who has quit smoking within the past 15 years
The United States Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with Low Dose Computed Tomography in adult individuals, between ages 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.  Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. This was a Grade: B recommendation which meant that the USPSTF recommends the service and there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. This therefore meant that clinicians offer or provide this service to these high risk individuals.
Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. This was a Grade: B recommendation which meant that the USPSTF recommends the service and there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. This therefore meant that clinicians offer or provide this service to these high risk individuals.
Based on this information the Centers for Medicare & Medicaid Services (CMS) on November 14, 2014, proposed that the evidence is sufficient, to add a Lung cancer screening counseling and shared decision making visit. CMS proposed, screening for Lung cancer with Low Dose Computed Tomography (LDCT), for appropriate beneficiaries, once per year, as an additional preventive service benefit under the Medicare program, only if all of the following criteria are met:
1. Age 55-74 years
2. Asymptomatic (no signs or symptoms of lung disease)
3. Tobacco smoking history of at least 30 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes)
4. Current smoker or one who has quit smoking within the last 15 years
5. A lung cancer screening counseling and shared decision making visit which includes the use of one or more decision aids discussing the benefits, harms, follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure
6. Counseling on the importance of adherence to annual LDCT lung cancer screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment
7. Counseling on the importance of maintaining cigarette smoking abstinence if former smoker, or smoking cessation if current smoker and, if appropriate, offering additional Medicare-covered tobacco cessation counseling services
Lung Cancer screening is performed using a non-contrast, Low Dose CT scan (LDCT) at an accredited advanced diagnostic imaging center with an effective radiation dose less than 1.5 mSv (the equivalent of 15 chest x-rays), compared to a standard chest CT with a median radiation dose of 8 mSv. The imaging center must collect and submit required data to a CMS-approved national registry for each LDCT lung cancer screening performed. Moyer VA, et al. on behalf of the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:330-338.
Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), 25% are Squamous cell carcinomas, 40% are Adenocarcinomas and 10% are Large cell carcinomas. With a better understanding of the Immune checkpoints, the gates are now wide open for the development of various immunotherapies. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. Checkmate -063 is a Phase II single arm, open label study designed to evaluate the efficacy of OPDIVO® (Nivolumab) in patients with advanced NSCLC with squamous histology, who had progressed on platinum based therapy as well as at least one additional systemic therapy. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody, which demonstrated an objective response in 20% – 25% of patients with advanced Non Small Cell Lung Cancer, Melanoma and Renal Cell Carcinoma, with favorable toxicities, in previously published studies. This study enrolled 117 patients and two thirds of the patients had previously failed 3 or more treatments and three fourths of patients were within 3 months of completion of their most recent therapy. OPDIVO® was administered as a single agent at 3mg/kg by intravenous infusion every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were overall survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% as assessed by an independent review committee and the median duration of response was not reached. These responses were independent of PD-L1 status for patients with quantifiable PD-L1 expression. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. Approximately 17% of the patients experienced grade 3-4 adverse events which included fatigue, pneumonitis and diarrhea. The authors concluded that the high response rates, median duration of response and disease control rates for Squamous NSCLC, is very promising in this difficult to treat group of patients and phase III trials are underway evaluating OPDIVO® monotherapy in frontline and previously treated patients with Non Small Cell Lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; October 30-November 1, 2014; Chicago, IL. LBA#3462
