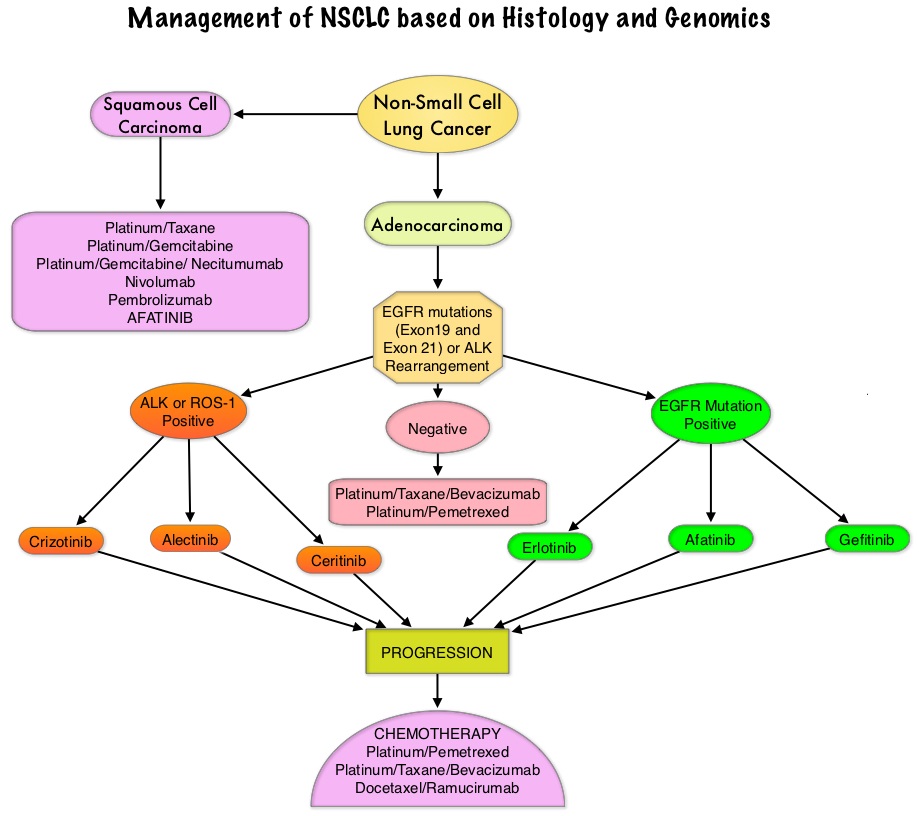
SUMMARY: The FDA on September 29, 2016 approved a blood-based companion diagnostic for TAGRISSO® (Osimertinib). The companion diagnostic for TAGRISSO® is the only FDA approved and clinically validated companion diagnostic test that uses either tissue or a blood sample to confirm the presence of a T790M point mutation in patients with metastatic Epidermal Growth Factor Receptor (EGFR) mutation-positive Non Small Cell Lung Cancer (NSCLC), who have had progression of disease on or after EGFR Tyrosine Kinase Inhibitor therapy. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large cell carcinomas. Approximately 10% to 15% of Caucasian patients and 50% of Asian patients with Adenocarcinomas, harbor activating EGFR mutations and 90% of these mutations are either Exon 19 deletions or L858R point mutations in Exon 21. EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60% to 70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9 to 14 months. This resistance to frontline EGFR TKI therapy has been attributed to acquired T790M “gatekeeper” point mutation in EGFR, identified in 50% – 60% of patients.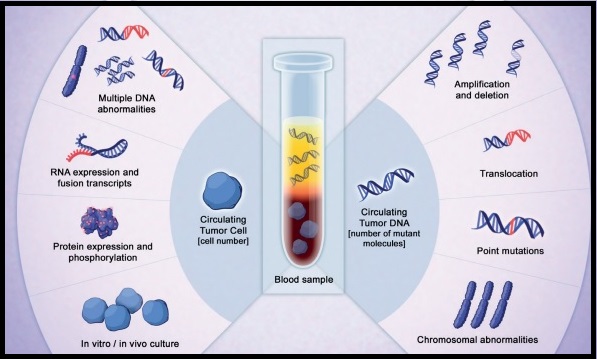
TAGRISSO® is presently approved by the FDA for the treatment of patients with metastatic EGFR T790M mutation-positive NSCLC, who had progressed on prior systemic therapy, including an EGFR TKI. The application of precision medicine with targeted therapy requires detection of molecular abnormalities in a tumor specimen, following progression or recurrence. Archived biopsy specimens may not be helpful as it is important to identify additional mutations in the tumor at the time of recurrence or progression, in order to plan appropriate therapy. Further, recurrent tumors may be inaccessible for a safe biopsy procedure or the clinical condition of the patient may not permit a repeat biopsy. Additionally, the biopsy itself may be subject to sampling error due to tumor heterogeneity. Genotyping circulating-free tumor DNA (cfDNA) in the plasma can potentially overcome the shortcomings of repeat biopsies and tissue genotyping, allowing the detection of many more targetable gene mutations, thus resulting in better evaluation of the tumor genome landscape.
The COBAS® Mutation Test v2, is a real-time PCR test for the qualitative detection of defined mutations of the EGFR gene in NSCLC patients. Defined EGFR mutations are detected using DNA isolated from Formalin-Fixed Paraffin-Embedded Tumor tissue (FFPET) or circulating-free tumor DNA (cfDNA) from plasma, obtained from EDTA anti-coagulated peripheral whole blood (purple top tube). This new blood-based companion diagnostic test offers an important option to identify T790M mutation in patients with metastatic EGFR mutation-positive NSCLC, who have progressed on an EGFR TKI therapy, and for whom a tissue biopsy may not be feasible.
US FDA approves Tagrisso (osimertinib) blood-based T790M companion diagnostic test. AstraZeneca website. https://www.astrazeneca-us.com/content/az-us/media/press-releases/2016/us-fda-approves-tagrisso-osimertinib-blood-based-t790m-companion-diagnostic-test-09292016.html. Updated September 29, 2016.