The FDA on March 30, 2017 granted regular approval to TAGRISSO®, for the treatment of patients with metastatic Epidermal Growth Factor Receptor (EGFR) T790M mutation-positive Non-Small Cell Lung Cancer (NSCLC), as detected by an FDA-approved test, whose disease has progressed on or after EGFR Tyrosine Kinase Inhibitor (TKI) therapy. TAGRISSO® is marketed by AstraZeneca Pharmaceuticals, LP.
Tag: Lung Cancer: Non-Small Cell
FDA Approves ALUNBRIG® for ALK Positive Non Small Cell Lung Cancer
SUMMARY: The FDA on April 28, 2017 granted accelerated approval to ALUNBRIG® (Brigatinib),for the treatment of patients with metastatic Anaplastic Lymphoma Kinase (ALK)-positive Non Small Cell Lung Cancer (NSCLC), who have progressed on or are intolerant to XALKORI® (Crizotinib). Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 222,500 new cases of lung cancer will be diagnosed in the United States in 2017 and over 155,870 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 25% are Squamous Cell Carcinomas, 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. The discovery of rearrangements of the Anaplastic Lymphoma Kinase (ALK) gene in some patients with advanced NSCLC and adenocarcinoma histology, led to the development of agents such as XALKORI® (Crizotinib), ZYKADIA® (Ceritinib), ALECENSA® (Alectinib) and now ALUNBRIG® (Brigatinib), with promising results. It has become clear that appropriate, molecularly targeted therapy for tumors with a molecular abnormality, results in the best outcomes. According to the US Lung Cancer Mutation Consortium (LCMC), two thirds of patients with advanced adenocarcinoma of the lung, have a molecular driver abnormality. The most common oncogenic drivers in patients with advanced adenocarcinoma of the lung are, KRAS in 25%, EGFR in 21% and ALK in 8% as well as other mutations in BRAF, HER2, AKT1 and fusions involving RET and ROS oncogenes. These mutations are mutually exclusive, and the presence of two simultaneous mutations are rare.
The approval was based on findings from the ALTA trial, which is a pivotal, open-label multicenter study in which 222 patients were randomized 1:1 ratio to receive ALUNBRIG® 90 mg orally once daily (N=112) or 180 mg once daily following a 7-day lead-in at 90 mg orally once daily (N=110). Two dose regimens were evaluated in this study, as clinical responses and Adverse Events varied with starting dose of ALUNBRIG®, in a previous Phase I/II study. Eligible patients had locally advanced or metastatic ALK-positive NSCLC, who had progressed on XALKORI®. The median age of patients across the study was 54 years and 67% of the patients had brain metastases. Patients were stratified by presence of brain metastases at baseline and best response to prior treatment with XALKORI®. The Primary endpoint was Objective Response Rate (ORR) and Secondary endpoints included Progression Free Survival (PFS) and CNS response.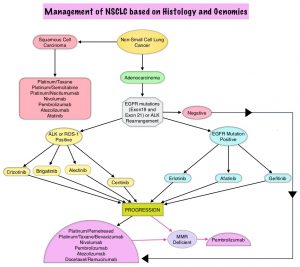
The ORR was 48% in the 90 mg dose group and 53% in the 180 mg dose group. After a median duration of follow up of 8 months, median duration of response was 13.8 months in both treatment groups. In patients with measurable brain metastases at baseline, intracranial ORR was 42% in the 90 mg group and 67% in the 180 mg group. Among patients who exhibited an intracranial response, 78% of patients in the 90 mg group and 68% of patients in the 180 mg group maintained an intracranial response for at least 4 months. The median PFS was 8.8 months in the 90 mg dose group, and 11.1 months in the 180mg dose group. The most common adverse reactions, were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and pneumonitis.
The authors concluded that treatment with ALUNBRIG® resulted in significant response rates and improved Progression Free Survival, with acceptable toxicity profile. The recommended dosing regimen of ALUNBRIG® is 90 mg orally once daily for the first 7 days and if tolerated, the dose is increased to 180 mg orally once daily.
Kim D-W, Tiseo M, Ahn M-J, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): First report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). J Clin Oncol. 2016;34 (suppl; abstr 9007).
Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening
SUMMARY: The rationale for Lung Cancer screening is based on the National Lung Cancer Screening Trial (NLST) in which the use of low dose CT scan in high risk, healthy patients, resulted in a 20% reduction in lung cancer mortality, compared to screening with a chest x-ray. It is important that eligible people who are smokers participate in a smoking cessation program and quit smoking. Further, those eligible for screening should understand the limitations associated with any screening methodology and potential risks associated with procedures that may follow a false positive result.
Lung cancer screening is performed using a non-contrast low dose CT scan. Criteria for lung cancer screening include-
1) People 55-74 years of age with no signs or symptoms of lung cancer
2) Current or former smoker with a 30 pack year smoking history (Number of years smoked multiplied by the number of packs of cigarettes per day)
3) Current smokers are strongly urged to enter a smoking cessation program
4) Former smokers must have quit smoking within the past 15 years
People with serious comorbid conditions, those on home oxygen and individuals with metallic devices or implants in the chest or back (which can interfere with the scan) should be excluded from lung cancer screening. Lung cancer screening with low dose CT scan is presently not covered by most insurance plans. The National Lung Screening Trial Research Team. N Engl J Med 2011; 365:395-409
Patients with Lung Cancer and Liver Metastases May Not Benefit from OPDIVO®
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers. The treatment paradigm for malignancies has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are now available that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1), that are expressed by cells in the tumor micro environment. By targeting the Immune check point proteins or their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.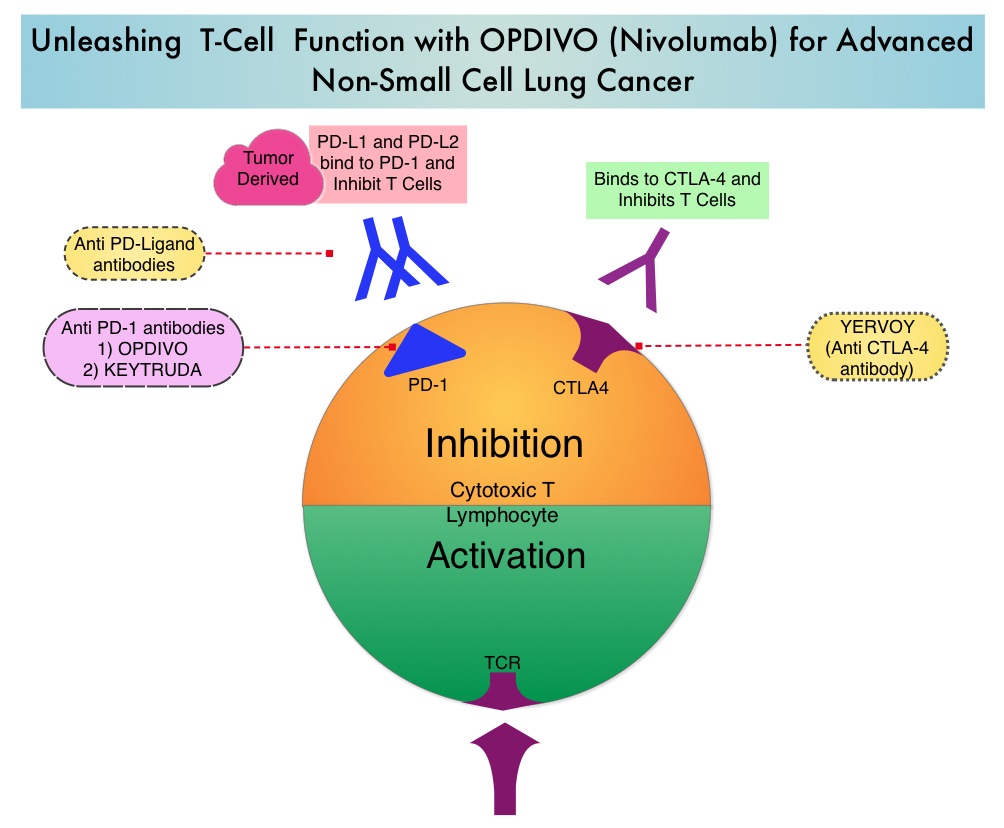
OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. Even though immune checkpoint inhibition has taken center stage in the management of advanced lung cancer as well as a number of other malignancies, the efficacy of these agents appears to vary based on the site of metastatic disease. Previously published studies have shown that in patients with advanced NSCLC, adrenal lesions and lymph nodes were more responsive when treated with OPDIVO®, followed by lung lesions. Liver lesions were less responsive to OPDIVO®. (Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. Journal for Immunotherapy of Cancer. 2016;4:84. doi:10.1186/s40425-016-0193-2).
To address this further, the authors in this publication reviewed the efficacy of OPDIVO® in lung cancer patients with hepatic metastases, at their institution. A retrospective study was conducted and this analysis included data from 75 patients with advanced lung cancer, who were treated with PD1 inhibitor OPDIVO®, at East Carolina University. The study was designed to evaluate predictive markers of response to immune checkpoint blockade. Thirteen percent (13%) of the patients had liver metastases. The median age was 62 years, 22% of the patients had squamous cell, 33% had adenocarcinoma, and 44% had small cell neuroendocrine histology. Patients had an average of 1.7 therapies prior to treatment with OPDIVO®. Patients in this study received an average of 4 cycles of anti-PD1 therapy with OPDIVOreg;. Forty four percent (44%) of the patients received adjunctive therapy such as radiation (33%) or immune modulating chemotherapy, with the aim of augmenting the effect of the anti-PD1 therapy.
It was noted that that none of the patients with hepatic metastases experienced an objective decrease in their liver metastases after treatment with OPDIVO®. These patients had an average survival of 132 days after initial treatment with OPDIVO®.
The authors concluded that this is the largest reported series evaluating lung cancer patients with hepatic metastases, who had been treated with PD-1 inhibitors. They noted that consistent with previously published studies, patients with liver metastases have poor outcomes with anti-PD1 therapy and the mechanisms underlying such resistance must be elucidated, so that more effective treatment combinations can be developed. Outcomes with immune checkpoint inhibitor use in lung cancer patients with hepatic metastases. Addepalli S, Chipman R, Stroud G, et al. J Clin Oncol 35, 2017 (suppl 7S; abstract 38)
First Line KEYTRUDA® Superior to Chemotherapy in Advanced NSCLC
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2017 about 222,500 new cases of lung cancer will be diagnosed and over 155,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers. The FDA in October, 2016 approved KEYTRUDA® (Pembrolizumab) for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors have high PD-L1 expression (Tumor Proportion Score greater than or equal to 50%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC.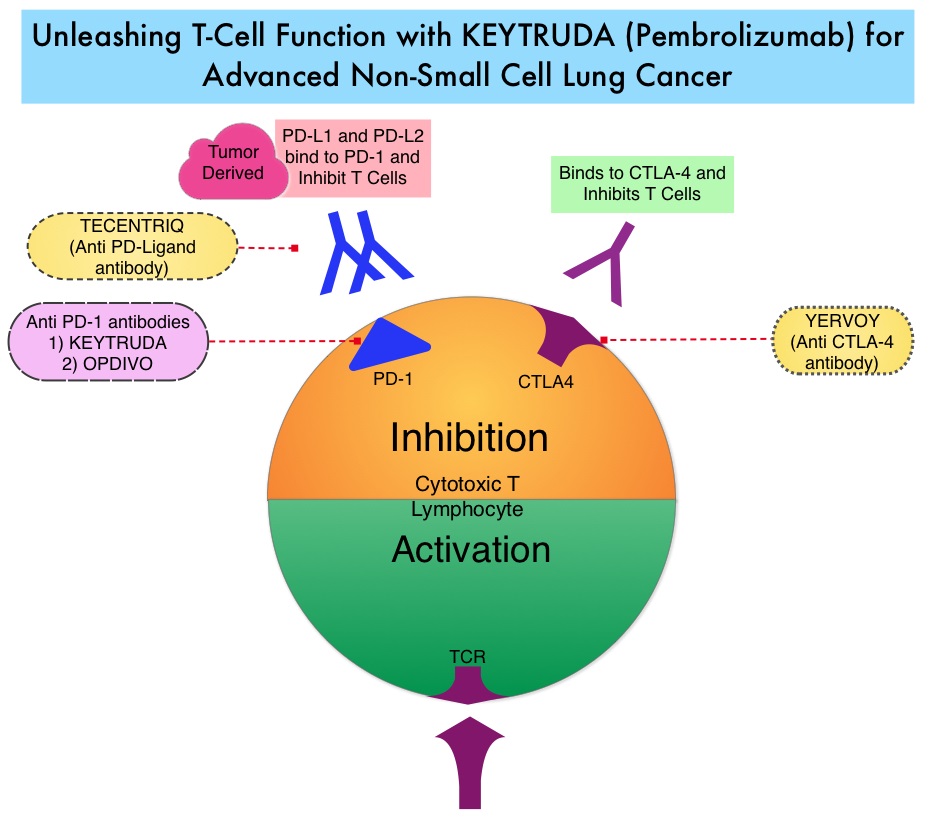
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced Non Small Cell Lung Cancer (NSCLC) have a high level of PD-L1 expression and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®.
KEYNOTE-024 is an open-label, randomized, phase III trial in which KEYTRUDA® administered at a fixed dose was compared with investigator’s choice of cytotoxic chemotherapy, as first line therapy, for patients with advanced NSCLC, with tumor PD-L1 expression of 50% or greater. Three hundred and five (N=305) treatment naïve patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, were randomly assigned in a 1:1 ratio to receive either KEYTRUDA® (N=154) or chemotherapy (N=151). Enrolled patients had no sensitizing EGFR mutations or ALK translocations. Treatment consisted of KEYTRUDA® administered at a fixed dose of 200 mg IV every 3 weeks for 35 cycles or the investigator’s choice of platinum-based chemotherapy for 4-6 cycles. Pemetrexed (ALIMTA®) based therapy was permitted only for patients who had non-squamous tumors and these patients could receive ALIMTA® maintenance therapy after the completion of combination chemotherapy. The primary end point was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety.
The median PFS was 10.3 months in the KEYTRUDA® group versus 6.0 months in the chemotherapy group (HR=0.50; P<0.001). This benefit was observed across all patient subgroups including tumor histologic type and chemotherapy regimen administered. The estimated Overall Survival at 6 months was 80.2% in the KEYTRUDA® group versus 72.4% in the chemotherapy group (HR=0.60; P=0.005). Patients in the KEYTRUDA® group experienced higher Response Rates than in the chemotherapy group (44.8% vs. 27.8%) as well as longer median duration of response (Not Reached versus 6.3 months). These benefits were realized even after 43.7% of the patients in the chemotherapy group following progression, had crossed over to receive KEYTRUDA®. Adverse events of any grade were less frequent in the KEYTRUDA® group compared to the chemotherapy group, with diarrhea, fatigue and pyrexia being more common in the KEYTRUDA® group whereas anemia, nausea and fatigue were more often noted in the chemotherapy group. As expected, immune-mediated adverse events (including pneumonitis) occurred more frequently with KEYTRUDA® whereas cytopenias occurred more frequently with chemotherapy.
It was concluded that in treatment naïve patients with advanced NSCLC and a PD-L1 tumor proportion score of 50% or greater, KEYTRUDA® was associated with significantly longer Progression Free and Overall Survival and with fewer adverse events, compared with platinum-based chemotherapy. This landmark trial is practice changing for advanced NSCLC. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. Reck M, Rodríguez-Abreu D, Robinson AG, et al. for the KEYNOTE-024 Investigators. October 9, 2016DOI: 10.1056/NEJMoa1606774
FDA Approves TECENTRIQ® for Non-Small Cell Lung Cancer
SUMMARY: The FDA on October 18, 2016, approved TECENTRIQ® (Atezolizumab) for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose disease progressed during or following platinum-containing chemotherapy. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. The treatment paradigm for malignancies has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are now available that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1), that are expressed by cells in the tumor micro environment. By targeting the Immune check point proteins or their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.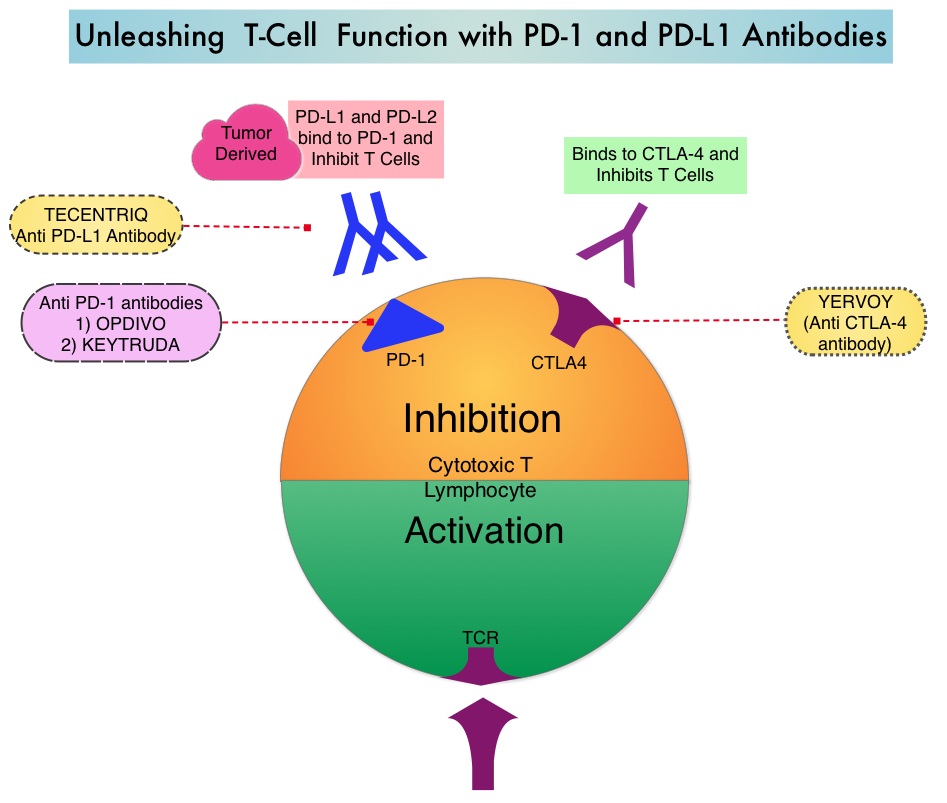
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating Immune Cells, thereby blocking its interactions with PD-1 and B7.1 receptors and thus enabling the activation of T cells and restoring tumor-specific T-cell immunity. The approval of TECENTRIQ® was based two international, clinical trials (OAK and POPLAR trials). TECENTRIQ® demonstrated survival benefit compared to Docetaxel in a multicenter, randomized, phase II study (POPLAR trial). In this study, the median Overall Survival was 12.6 months and 9.7 months (HR=0.69) for the TECENTRIQ® and Docetaxel groups respectively.
OAK trial is a global, multicentre, open-label, randomized, controlled Phase III study in which 1225 patients with locally advanced or metastatic NSCLC, whose disease had progressed following previous treatment with platinum-containing chemotherapy, were enrolled. Patients with both squamous and non-squamous histology were randomized in a 1:1 ratio to receive either TECENTRIQ® 1200 mg IV every 3 weeks or Docetaxel 75 mg/m2 IV every 3 weeks. Patients were stratified according to PD-L1 status, number of prior chemotherapy regimens and histology. The median age was 64 years, 25% had 2 prior lines of therapy and 26% had squamous histology. The co-primary endpoints were Overall Survival (OS) in all randomized patients and in a PD-L1 selected subgroup in the primary analysis population. Secondary endpoints included Progression Free Survival, Objective Response Rate, Duration of Response and Safety.
The primary efficacy analysis was conducted and reported in the first 850 of 1225 total enrolled patients. The median Overall Survival was 13.8 months in the TECENTRIQ® group compared to 9.6 months in the Docetaxel group (HR=0.74; P=0.0004), with a 26% improvement in Overall Survival in the patient group who received TECENTRIQ®. This benefit was seen regardless of their PD-L1 expression levels, including patients whose tumors displayed PD-L1 expression of less than 1%. Patients with high PD-L1 expression had more pronounced benefit with TECENTRIQ® with a 59% improvement in OS compared with Docetaxel (HR=0.41; P<0.0001). The OS benefit was similar in patients with squamous or non-squamous histology. The most common adverse reactions in patients in patients treated with TECENTRIQ® were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation.
The authors concluded that TECENTRIQ® offers a new second-line therapeutic strategy for patients with Non Small Cell Lung Cancer, with Overall Survival benefit, regardless of the PD-L1 status of the tumor. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Barlesi F, Park K, Ciardiello F et al. Abstract LBA44_PR. Presented at: 2016 ESMO Congress; October 7–11 (2016) Copenhagen, Denmark.
FDA Approves KEYTRUDA® for Treatment Naïve Patients with Advanced NSCLC
SUMMARY: The FDA on October 24, 2016 approved KEYTRUDA® (Pembrolizumab) for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors have high PD-L1 expression (Tumor Proportion Score greater than or equal to 50%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment for metastatic NSCLC. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer accounts for approximately 85% of all lung cancers.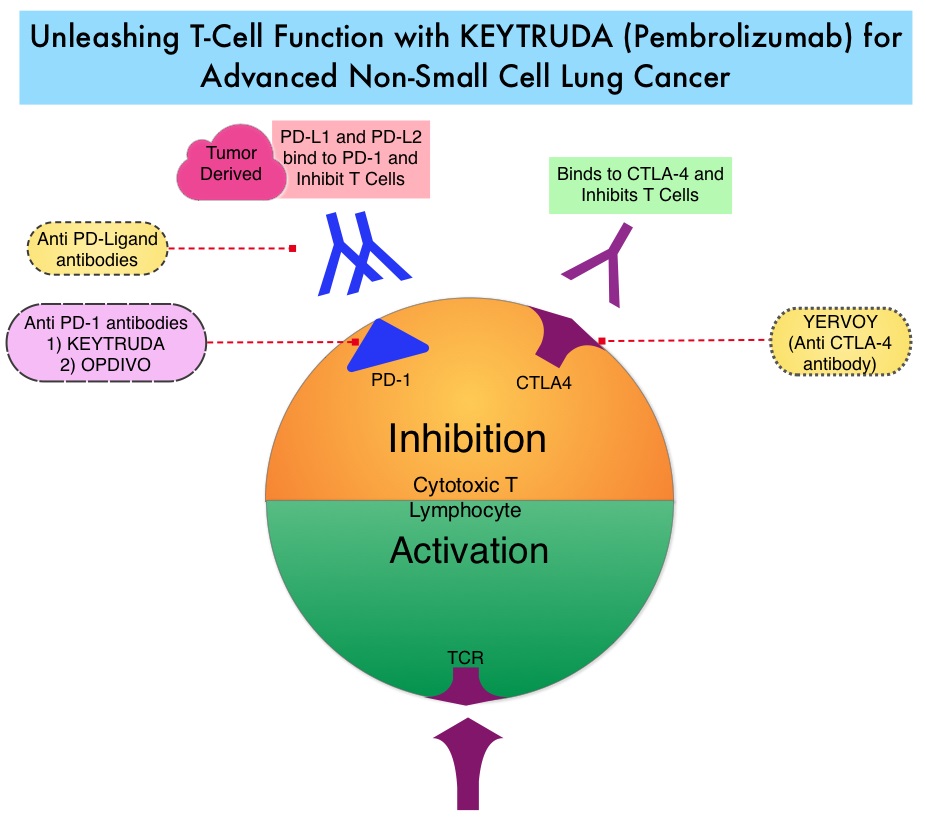
KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced Non Small Cell Lung Cancer (NSCLC) have a high level of PD-L1 expression and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®.
KEYNOTE-024 is a open-label, randomized, phase III trial in which KEYTRUDA® administered at a fixed dose was compared with investigator’s choice of cytotoxic chemotherapy, as first line therapy, for patients with advanced NSCLC, with tumor PD-L1 expression of 50% or greater. Three hundred and five (N=305) treatment naïve patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, were randomly assigned in a 1:1 ratio to receive either KEYTRUDA® (N=154) or chemotherapy (N=151). Enrolled patients had no sensitizing EGFR mutations or ALK translocations. Treatment consisted of KEYTRUDA® administered at a fixed dose of 200 mg IV every 3 weeks for 35 cycles or the investigator’s choice of platinum-based chemotherapy for 4-6 cycles. Pemetrexed (ALIMTA®) based therapy was permitted only for patients who had non-squamous tumors and these patients could receive ALIMTA® maintenance therapy after the completion of combination chemotherapy. The primary end point was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety.
The median PFS was 10.3 months in the KEYTRUDA® group versus 6.0 months in the chemotherapy group (HR=0.50; P<0.001). This benefit was observed across all patient subgroups including tumor histologic type and chemotherapy regimen administered. The estimated Overall Survival at 6 months was 80.2% in the KEYTRUDA® group versus 72.4% in the chemotherapy group (HR=0.60; P=0.005). Patients in the KEYTRUDA® group experienced higher Response Rates than in the chemotherapy group (44.8% vs. 27.8%) as well as longer median duration of response (Not Reached versus 6.3 months). These benefits were realized even after 43.7% of the patients in the chemotherapy group following progression, had crossed over to receive KEYTRUDA®. Adverse events of any grade were less frequent in the KEYTRUDA® group compared to the chemotherapy group, with diarrhea, fatigue and pyrexia being more common in the KEYTRUDA® group whereas anemia, nausea and fatigue were more often noted in the chemotherapy group. As expected, immune-mediated adverse events (including pneumonitis) occurred more frequently with KEYTRUDA® whereas cytopenias occurred more frequently with chemotherapy.
It was concluded that in treatment naïve patients with advanced NSCLC and a PD-L1 tumor proportion score of 50% or greater, KEYTRUDA® was associated with significantly longer Progression Free and Overall Survival and with fewer adverse events, compared with platinum-based chemotherapy. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. Reck M, Rodríguez-Abreu D, Robinson AG, et al. for the KEYNOTE-024 Investigators. October 9, 2016DOI: 10.1056/NEJMoa1606774
KEYTRUDA® (Pembrolizumab)
The FDA on October 24, 2016 approved KEYTRUDA® for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC), whose tumors express PD-L1, as determined by an FDA-approved test. This is the first FDA approval of a checkpoint inhibitor for first-line treatment of Lung cancer. This approval also expands the indication in second-line treatment of Lung cancer to include all patients with PD-L1-expressing NSCLC. KEYTRUDA® is a product of Merck & Co., Inc.
TECENTRIQ® (Atezolizumab)
The FDA on October 18, 2016 approved TECENTRIQ® for the treatment of patients with metastatic Non Small Cell Lung Cancer (NSCLC) whose disease progressed during or following Platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations, prior to receiving TECENTRIQ®. TECENTRIQ® is a product of Genentech, Inc.
TARCEVA® (Erlotinib)
The FDA on October 18, 2016 modified the indication for TARCEVA® for treatment of Non Small Cell Lung Cancer, to limit use to patients whose tumors have specific Epidermal Growth Factor Receptor (EGFR) mutations. TARCEVA® is a product of Astellas Pharm Global Development Inc.
