Written by Thomas Hutson, D.O., Pharm. D.
Sponsored by Exelixis, Inc.
At the 2023 GU Cancer Symposium jointly sponsored by ASCO, ASTRO, and SUO, Dr Maurice Burotto presented the minimum 3-year follow-up efficacy and safety data for the ITT population in the Phase 3 CheckMate-9ER trial. These extended follow-up analysis results continue to support cabozantinib + nivolumab as a first-line treatment for patients with advanced RCC.1,2
CheckMate‐9ER was a randomized (1:1), open‐label, Phase 3 trial vs sunitinib in 651 patients with previously untreated aRCC with a clear‐cell component.1,3
- Dosing: cabozantinib 40 mg (starting dose) PO once daily in combination with nivolumab 240 mg flat dose IV every 2 weeks vs sunitinib 50 mg (starting dose) PO once daily for 4 weeks, followed by 2 weeks off, per cycle.1
- The starting dose of cabozantinib is 40 mg when used in combination with nivolumab, unlike the 60‐mg recommended starting dose for single‐agent therapy1
- Endpoints assessed1,3-6:
- Primary endpoint was PFS*
- Secondary endpoints were OS, ORR,* and safety
- Quality of life: evaluated as an exploratory endpoint using the FKSI‐19 scale, and the clinical significance of the results is unknown
- Additional exploratory endpoints: biomarkers, pharmacokinetics, immunogenicity, and PFS‐2
- Updated efficacy analysis: conducted when 271 events were observed based on the pre‐specified number of events for the pre‐planned final analysis of OS1,7,8
- Patient population in CheckMate-9ER was representative of the aRCC population seen in clinical practice (cabozantinib + nivolumab, n=323; sunitinib, n=328)1,3,4,9-11
- IMDC risk categories3
- Favorable: 23% of cabozantinib + nivolumab patients; 22% of sunitinib patients
- Intermediate: 58% of cabozantinib + nivolumab patients; 57% of sunitinib patients
- Poor: 19% of cabozantinib + nivolumab patients; 21% of sunitinib patients
- Prior nephrectomy: 69% of cabozantinib + nivolumab patients; 71% of sunitinib patients9
- Liver metastases: 23% of cabozantinib + nivolumab patients; 16% of sunitinib patients3
- Bone metastases: 24% of cabozantinib + nivolumab patients; 22% of sunitinib patients3
- ≥2 metastatic sites: 80% of cabozantinib + nivolumab patients; 78% of sunitinib patients3
- IMDC risk categories3
Primary analysis results (median follow‐up time of 18.1 months; range: 10.6‐30.6 months)3:
- Median PFS was 16.6 months for cabozantinib + nivolumab (95% CI: 12.5-24.9; n=323) compared with 8.3 months for sunitinib (95% CI: 7.0-9.7); HR=0.51 (95% CI: 0.41-0.64)1*
- Probability of OS at 12 months was 85.7% for cabozantinib + nivolumab (95% CI: 81.3-89.1) compared with 75.6% for sunitinib (95% CI: 70.5-80.0); HR=0.60 (98.89% CI: 0.40-0.89). The median OS was not reached in either group3
- ORR was 55.7% for cabozantinib + nivolumab (95% CI: 50.1-61.2; n=323) compared with 27.1% for sunitinib (95% CI: 22.4-32.3; n=328)1*
Updated pre-planned analysis of OS (median follow‐up: 32.9 months; range: 25.4‐45.4 months)1,7,8:
- Median OS was 37.7 months for cabozantinib + nivolumab (95% CI: 35.5‐NR; n=323) compared with 34.3 months for sunitinib (95% CI: 29.0‐NR; n=328); HR=0.70 (95% CI: 0.55‐0.90)
More than 2 years of additional follow-up from the primary analysis, which makes it the longest available for CheckMate-9ER (median follow‐up: 44 months; range: 36.5-56.5 months):2
- Median PFS was 16.6 months for cabozantinib + nivolumab (95% CI: 12.8-19.5; n=323) compared with 8.4 months for sunitinib (95% CI: 7.0-9.7; n=328); HR=0.59 (95% CI: 0.49-0.71)2
- ORR was 56.0% for cabozantinib + nivolumab (95% CI: 50.4-61.5; n=323) compared with 28.0% for sunitinib (95% CI: 23.3-33.2; n=328)2
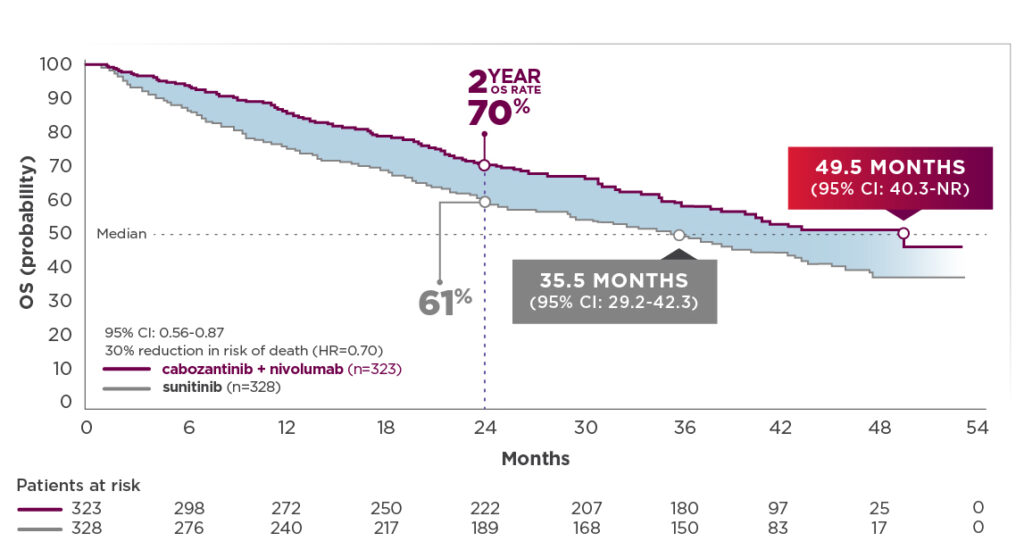
- Median OS (depicted in graph below) was 49.5 months for cabozantinib + nivolumab (95% CI: 40.3-NR; n=323) compared with 35.5 months for sunitinib (95% CI: 29.2-42.3); HR=0.70 (95% CI: 0.56-0.87)2,12
- The median OS for cabozantinib + nivolumab patients was 14 months longer than the sunitinib arm.2,12 This substantial difference was derived without adjustment for subsequent cancer therapy3
44-month follow-up analysis2,12
OS: Median follow-up time of 44.0 months; range: 36.5-56.5 months
PFS and ORR results in patients with bone, liver, and/or lung metastasis shown below13:
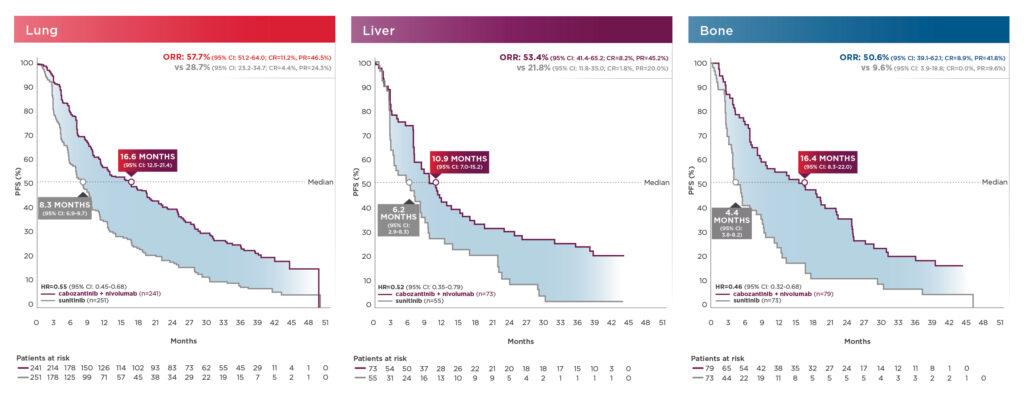
These exploratory analyses are descriptive in nature. Subgroups were not powered to show differences between treatment arms, and results should be considered hypothesis generating.7
In CheckMate-9ER, serious adverse reactions occurred in 48% of patients receiving cabozantinib + nivolumab.1 Serious adverse reactions reported in ≥2% of patients were diarrhea, pneumonia, pneumonitis, pulmonary embolism, urinary tract infection, and hyponatremia.1 Fatal intestinal perforations occurred in 3 (0.9%) patients.1 The most common adverse reactions (≥20%) in patients receiving cabozantinib + nivolumab (n=320) vs sunitinib (n=320) were diarrhea (64% vs 47%), fatigue (51% vs 50%), hepatotoxicity (44% vs 26%), palmar‐plantar erythrodysesthesia (40% vs 41%), stomatitis (37% vs 46%), rash (36% vs 14%), hypertension (36% vs 39%), hypothyroidism (34% vs 30%), musculoskeletal pain (33% vs 29%), decreased appetite (28% vs 20%), nausea (27% vs 31%), dysgeusia (24% vs 22%), abdominal pain (22% vs 15%), upper respiratory tract infection (20% vs 8%), and cough (20% vs 17%).1
- Cabozantinib may be interrupted or reduced due to adverse events to 20 mg daily or 20 mg every other day.1 The average dosage of cabozantinib in CheckMate-9ER was 30 mg14
- If previously receiving 20 mg once every other day, resume at same dosage. If not tolerated, discontinue cabozantinib1
- Adverse reactions leading to discontinuation of either cabozantinib or nivolumab occurred in 20% of patients, which included 8% for only cabozantinib and 7% for only nivolumab.3 It is important to note that 6% of patients in the CheckMate‐9ER trial discontinued both cabozantinib and nivolumab at the same time due to adverse events, compared with 17% of patients in the sunitinib arm who permanently discontinued their treatment3
- Cabozantinib should be permanently discontinued for Grade 3 or 4 hemorrhage, development of a GI perforation or Grade 4 fistula, acute myocardial infarction or Grade 2 or higher cerebral infarction, Grade 3 or 4 arterial thromboembolic events or Grade 4 venous thromboembolic events, Grade 4 hypertension/hypertensive crisis or Grade 3 hypertension/hypertensive crisis that cannot be controlled, nephrotic syndrome, or reversible posterior leukoencephalopathy syndrome1
- For patients being treated with cabozantinib + nivolumab, if ALT or AST >10x ULN or >3x ULN with concurrent total bilirubin ≥2x ULN, both cabozantinib + nivolumab should be permanently discontinued1
In summary, the 44-month follow-up data indicate that:
- After a minimum follow-up of 3 years, survival and response benefits were maintained with cabozantinib + nivolumab, showing consistent outcomes as in previous follow-ups1,2
- Median OS with cabozantinib + nivolumab improved by 11.8 months since the previous follow-up analysis. Median OS with cabozantinib + nivolumab was 49.5 months compared with 35.5 months for sunitinib1,2,7,8,12
- The median OS for cabozantinib + nivolumab patients was substantially longer (14 months) than the sunitinib arm. This difference was derived without adjustment for subsequent cancer therapy2,3
- No new safety signals emerged with additional follow-up in either arm2
- Among patients treated with cabozantinib + nivolumab, the discontinuation rate due to ARs to cabozantinib alone was 10% and nivolumab alone was 10%, vs 11% for sunitinib.2 This may allow patients receiving cabozantinib + nivolumab to stay on therapy and thus allow them to achieve efficacy benefits
Dr Hutson received a fee for participating in this program, and his comments reflect his opinions and are not intended to constitute medical advice for individual patients.
[Footnotes]
*PFS and ORR were assessed by BICR.1
1L=first‐line; ALT=alanine aminotransferase; AR=adverse reaction; aRCC=advanced RCC; ASCO=American Society of Clinical Oncology; AST=aspartate aminotransferase; ASTRO=American Society for Radiation Oncology; BICR=blinded independent central review; CI=confidence interval; CR=complete response; FKSI‐19=Functional Assessment of Cancer Therapy‐Kidney Symptom Index 19; HR=hazard ratio; IMDC=International Metastatic RCC Database Consortium; IV=intravenous; NR=not reached; ORR=objective response rate; OS=overall survival; PFS=progression‐free survival; PFS‐2=PFS after subsequent therapy; PO=by mouth; PR=partial response; RCC=renal cell carcinoma; SUO=Society of Urologic Oncology; ULN=upper limit of normal.
INDICATIONS
CABOMETYX® (cabozantinib) is indicated for the treatment of patients with advanced renal cell carcinoma (RCC).
CABOMETYX, in combination with nivolumab, is indicated for the first-line treatment of patients with advanced RCC.
CABOMETYX is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
CABOMETYX is indicated for the treatment of adult and pediatric patients 12 years of age and older with locally advanced or metastatic differentiated thyroid cancer (DTC) that has progressed following prior VEGFR-targeted therapy and who are radioactive iodine-refractory or ineligible.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Hemorrhage: Severe and fatal hemorrhages occurred with CABOMETYX. The incidence of Grade 3 to 5 hemorrhagic events was 5% in CABOMETYX patients in RCC, HCC, and DTC studies. Discontinue CABOMETYX for Grade 3 or 4 hemorrhage and prior to surgery as recommended. Do not administer CABOMETYX to patients who have a recent history of hemorrhage, including hemoptysis, hematemesis, or melena.
Perforations and Fistulas: Fistulas, including fatal cases, occurred in 1% of CABOMETYX patients. Gastrointestinal (GI) perforations, including fatal cases, occurred in 1% of CABOMETYX patients. Monitor patients for signs and symptoms of fistulas and perforations, including abscess and sepsis. Discontinue CABOMETYX in patients who experience a Grade 4 fistula or a GI perforation.
Thrombotic Events: CABOMETYX increased the risk of thrombotic events. Venous thromboembolism occurred in 7% (including 4% pulmonary embolism) and arterial thromboembolism in 2% of CABOMETYX patients. Fatal thrombotic events occurred in CABOMETYX patients. Discontinue CABOMETYX in patients who develop an acute myocardial infarction or serious arterial or venous thromboembolic events that require medical intervention.
Hypertension and Hypertensive Crisis: CABOMETYX can cause hypertension, including hypertensive crisis. Hypertension was reported in 37% (16% Grade 3 and <1% Grade 4) of CABOMETYX patients. Do not initiate CABOMETYX in patients with uncontrolled hypertension. Monitor blood pressure regularly during CABOMETYX treatment. Withhold CABOMETYX for hypertension that is not adequately controlled with medical management; when controlled, resume at a reduced dose. Permanently discontinue CABOMETYX for severe hypertension that cannot be controlled with anti-hypertensive therapy or for hypertensive crisis.
Diarrhea: Diarrhea occurred in 62% of CABOMETYX patients. Grade 3 diarrhea occurred in 10% of CABOMETYX patients. Monitor and manage patients using antidiarrheals as indicated. Withhold CABOMETYX until improvement to ≤ Grade 1, resume at a reduced dose.
Palmar-Plantar Erythrodysesthesia (PPE): PPE occurred in 45% of CABOMETYX patients. Grade 3 PPE occurred in 13% of CABOMETYX patients. Withhold CABOMETYX until improvement to Grade 1 and resume at a reduced dose for intolerable Grade 2 PPE or Grade 3 PPE.
Hepatotoxicity: CABOMETYX in combination with nivolumab can cause hepatic toxicity with higher frequencies of Grades 3 and 4 ALT and AST elevations compared to CABOMETYX alone.
Monitor liver enzymes before initiation of and periodically throughout treatment. Consider more frequent monitoring of liver enzymes than when the drugs are administered as single agents. For elevated liver enzymes, interrupt CABOMETYX and nivolumab and consider administering corticosteroids.
With the combination of CABOMETYX and nivolumab, Grades 3 and 4 increased ALT or AST were seen in 11% of patients. ALT or AST >3 times ULN (Grade ≥2) was reported in 83 patients, of whom 23 (28%) received systemic corticosteroids; ALT or AST resolved to Grades 0-1 in 74 (89%). Among the 44 patients with Grade ≥2 increased ALT or AST who were rechallenged with either CABOMETYX (n=9) or nivolumab (n=11) as a single agent or with both (n=24), recurrence of Grade ≥2 increased ALT or AST was observed in 2 patients receiving CABOMETYX, 2 patients receiving nivolumab, and 7 patients receiving both CABOMETYX and nivolumab. Withhold and resume at a reduced dose based on severity.
Adrenal Insufficiency: CABOMETYX in combination with nivolumab can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold CABOMETYX and/or nivolumab and resume CABOMETYX at a reduced dose depending on severity.
Adrenal insufficiency occurred in 4.7% (15/320) of patients with RCC who received CABOMETYX with nivolumab, including Grade 3 (2.2%), and Grade 2 (1.9%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of CABOMETYX and nivolumab in 0.9% and withholding of CABOMETYX and nivolumab in 2.8% of patients with RCC.
Approximately 80% (12/15) of patients with adrenal insufficiency received hormone replacement therapy, including systemic corticosteroids. Adrenal insufficiency resolved in 27% (n=4) of the 15 patients. Of the 9 patients in whom CABOMETYX with nivolumab was withheld for adrenal insufficiency, 6 reinstated treatment after symptom improvement; of these, all (n=6) received hormone replacement therapy and 2 had recurrence of adrenal insufficiency.
Proteinuria: Proteinuria was observed in 8% of CABOMETYX patients. Monitor urine protein regularly during CABOMETYX treatment. For Grade 2 or 3 proteinuria, withhold CABOMETYX until improvement to ≤ Grade 1 proteinuria, resume CABOMETYX at a reduced dose. Discontinue CABOMETYX in patients who develop nephrotic syndrome.
Osteonecrosis of the Jaw (ONJ): ONJ occurred in <1% of CABOMETYX patients. ONJ can manifest as jaw pain, osteomyelitis, osteitis, bone erosion, tooth or periodontal infection, toothache, gingival ulceration or erosion, persistent jaw pain, or slow healing of the mouth or jaw after dental surgery. Perform an oral examination prior to CABOMETYX initiation and periodically during treatment. Advise patients regarding good oral hygiene practices. Withhold CABOMETYX for at least 3 weeks prior to scheduled dental surgery or invasive dental procedures, if possible. Withhold CABOMETYX for development of ONJ until complete resolution, resume at a reduced dose
Impaired Wound Healing: Wound complications occurred with CABOMETYX. Withhold CABOMETYX for at least 3 weeks prior to elective surgery. Do not administer CABOMETYX for at least 2 weeks after major surgery and until adequate wound healing. The safety of resumption of CABOMETYX after resolution of wound healing complications has not been established.
Reversible Posterior Leukoencephalopathy Syndrome (RPLS): RPLS, a syndrome of subcortical vasogenic edema diagnosed by characteristic findings on MRI, can occur with CABOMETYX. Evaluate for RPLS in patients presenting with seizures, headache, visual disturbances, confusion, or altered mental function. Discontinue CABOMETYX in patients who develop RPLS.
Thyroid Dysfunction: Thyroid dysfunction, primarily hypothyroidism, has been observed with CABOMETYX. Based on the safety population, thyroid dysfunction occurred in 19% of patients treated with CABOMETYX, including Grade 3 in 0.4% of patients.
Patients should be assessed for signs of thyroid dysfunction prior to the initiation of CABOMETYX and monitored for signs and symptoms of thyroid dysfunction during CABOMETYX treatment. Thyroid function testing and management of dysfunction should be performed as clinically indicated.
Hypocalcemia: CABOMETYX can cause hypocalcemia. Based on the safety population, hypocalcemia occurred in 13% of patients treated with CABOMETYX, including Grade 3 in 2% and Grade 4 in 1% of patients. Laboratory abnormality data were not collected in CABOSUN.
In COSMIC-311, hypocalcemia occurred in 36% of patients treated with CABOMETYX, including Grade 3 in 6% and Grade 4 in 3% of patients.
Monitor blood calcium levels and replace calcium as necessary during treatment. Withhold and resume at reduced dose upon recovery or permanently discontinue CABOMETYX depending on severity.
Embryo-Fetal Toxicity: CABOMETYX can cause fetal harm. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Verify the pregnancy status of females of reproductive potential prior to initiating CABOMETYX and advise them to use effective contraception during treatment and for 4 months after the last dose.
ADVERSE REACTIONS
The most common (≥20%) adverse reactions are:
CABOMETYX as a single agent: diarrhea, fatigue, PPE, decreased appetite, hypertension, nausea, vomiting, weight decreased, constipation.
CABOMETYX in combination with nivolumab: diarrhea, fatigue, hepatotoxicity, PPE, stomatitis, rash, hypertension, hypothyroidism, musculoskeletal pain, decreased appetite, nausea, dysgeusia, abdominal pain, cough, and upper respiratory tract infection.
DRUG INTERACTIONS
Strong CYP3A4 Inhibitors: If coadministration with strong CYP3A4 inhibitors cannot be avoided, reduce the CABOMETYX dosage. Avoid grapefruit or grapefruit juice.
Strong CYP3A4 Inducers: If coadministration with strong CYP3A4 inducers cannot be avoided, increase the CABOMETYX dosage. Avoid St. John’s wort.
USE IN SPECIFIC POPULATIONS
Lactation: Advise women not to breastfeed during CABOMETYX treatment and for 4 months after the final dose.
Hepatic Impairment: In patients with moderate hepatic impairment, reduce the CABOMETYX dosage. Avoid CABOMETYX in patients with severe hepatic impairment.
Please see accompanying full Prescribing Information by clicking here.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.FDA.gov/medwatch or call 1-800-FDA-1088.
References
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Burotto M, Powles T, Escudier B, et al. Nivolumab plus cabozantinib versus sunitinib for first-line treatment of advanced renal cell carcinoma: 3-year follow-up from the phase 3 CheckMate 9ER trial. Poster presented at Cancer Immunotherapy and Immunomonitoring Conference; April 24-27, 2023.
- Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2021;384(9):829‐841.
- Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal cell carcinoma: outcomes by sarcomatoid histology and updated trial results with extended follow‐up of CheckMate 9ER. Poster presented at Genitourinary Cancers Symposium; February 11‐13, 2021
- Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma [supplementary appendix]. N Engl J Med. 2021;384(9):829-841.
- Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal‐cell carcinoma [protocol]. N Engl J Med. 2021;384(9):829‐841.
- Motzer RJ, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib in first‐line treatment for advanced renal cell carcinoma (CheckMate 9ER): long‐term follow‐up results from an open‐label, randomized, phase 3 trial. Lancet Oncol. 2022;23(7):888‐898.
- Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ‐specific target lesion assessments with 2‐year follow‐up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. Poster presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium; February 17‐19, 2022
- Data on file. Topline 9ER. Exelixis, Inc.
- Savard M-F, Wells JC, Graham J, et al. Real-world assessment of clinical outcomes among first-line sunitinib patients with clear cell metastatic renal cell carcinoma (mRCC) by the International mRCC Database Consortium risk group. Oncologist. 2020;25(5):422-430.
- Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794-5799.
- Data on file. Exelixis, Inc.
- Data on file. Exelixis, Inc.
- Data on file. Final Clinical Study Report for Study CA2099ER. Bristol Myers Squibb.
©2023 Exelixis, Inc. CA‐2644-1 07/23
OPDIVO® and the related logo are registered trademarks of Bristol‐Myers Squibb Company

